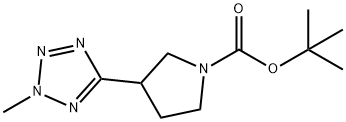
tert-butyl 3-(2-methyl-2H-tetrazol-5-yl)pyrrolidine-1-carboxylate synthesis
- Product Name:tert-butyl 3-(2-methyl-2H-tetrazol-5-yl)pyrrolidine-1-carboxylate
- CAS Number:1225218-83-6
- Molecular formula:C11H19N5O2
- Molecular Weight:253.3
1186299-03-5
6 suppliers
inquiry

74-88-4
351 suppliers
$15.00/10g

1225218-83-6
1 suppliers
inquiry
1225218-84-7
1 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium carbonate in acetonitrile; for 3 h;Reflux;
Steps:
B
Iodomethane (37.0 μL, 0.600 mmol) was added to a stirred solution of i-17a (120 mg, 0.500 mmol) and potassium carbonate (97.0 mg, 0.700 mmol) in acetonitrile (5.00 mL), and the resulting mixture was heated to reflux. After 3 h, the reaction mixture was cooled to rt, filtered and concentrated in vacuo. The crude residue was dissolved in DCM, and the organics were washed with water and brine, dried (sodium sulfate) and concentrated in vacuo to afford the title compound, i-17b, and regioisomer, i-17c. mlz (ES) 254 (MH)H .
References:
WO2010/51245,2010,A1 Location in patent:Page/Page column 51-52