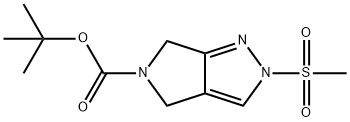
tert-butyl 2-(Methylsulfonyl)-4,6-dihydropyrrolo[3,4-c]pyrazole-5(2H)-carboxylate synthesis
- Product Name:tert-butyl 2-(Methylsulfonyl)-4,6-dihydropyrrolo[3,4-c]pyrazole-5(2H)-carboxylate
- CAS Number:1226781-82-3
- Molecular formula:C11H17N3O4S
- Molecular Weight:287.34
![tert-butyl 2-(Methylsulfonyl)-4,6-dihydropyrrolo[3,4-c]pyrazole-5(2H)-carboxylate tert-butyl 2-(Methylsulfonyl)-4,6-dihydropyrrolo[3,4-c]pyrazole-5(2H)-carboxylate](/NewsImg/2023-10-17/6383313814796791749926853.jpg)
![Pyrrolo[3,4-c]pyrazole-5(1H)-carboxylic acid, 4,6-dihydro-, 1,1-dimethylethyl ester](/CAS/GIF/657428-42-7.gif)
657428-42-7
144 suppliers
$75.00/250mg
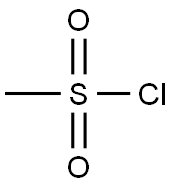
124-63-0
6 suppliers
$10.00/5g
![tert-butyl 2-(Methylsulfonyl)-4,6-dihydropyrrolo[3,4-c]pyrazole-5(2H)-carboxylate](/CAS/20150408/GIF/1226781-82-3.gif)
1226781-82-3
95 suppliers
$32.00/250mg
Yield:1226781-82-3 44%
Reaction Conditions:
Stage #1: 2,6-dihydropyrrole [3,4-c]pyrazole-5(4H)-carboxylic acid tert-butyl esterwith sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.5 h;
Stage #2: methanesulfonyl chloride in tetrahydrofuran;mineral oil at 20 - 30; for 1 h;
Steps:
3.1 Step 1:
tert-butyl 2-methylsulfonyl-4,6-dihydropyrrolo[3,4-c]pyrazole-5-carboxylate (3a)
Intermediate 2 (3.5 g, 16.7 mmol) was dissolved in tetrahydrofuran (35 ml), and sodium hydride (1.0 g, 60%, 25.4 mmol) was added at 0°C, followed by reaction for 30 min.
Methylsulfonyl chloride (2.9 g, 25.4 mmol) was added, followed by reaction for 1 hour.
The reaction was quenched by addition of water (10 ml) to the reaction solution, which was extracted with ethyl acetate (50 ml*2).
The organic layers were combined, dried over anhydrous sodium sulfate, concentrated, and purified by silica gel column chromatography (petroleum ether/ethyl acetate (v/v) = 1:1), to obtain a white solid 3a (2.1 g, yield 44%).
References:
EP3159344,2017,A1 Location in patent:Paragraph 0096; 0132; 0133