
8-bromo-7-(but-2-ynyl)-3-methyl-1H-purine-2,6(3H,7H)-dione synthesis
- Product Name:8-bromo-7-(but-2-ynyl)-3-methyl-1H-purine-2,6(3H,7H)-dione
- CAS Number:666816-98-4
- Molecular formula:C10H9BrN4O2
- Molecular Weight:297.11

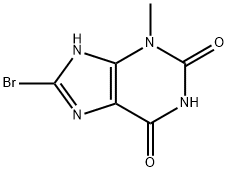
93703-24-3
393 suppliers
$30.00/1g

3355-28-0
369 suppliers
$6.00/1g

666816-98-4
391 suppliers
$19.00/1g
Yield:666816-98-4 98%
Reaction Conditions:
with sodium carbonate in acetone at 40; for 4 h;Temperature;Reagent/catalyst;Solvent;
Steps:
7-9 Example 7: Preparation of Compound IV
Compound III (2.450 g, 10 mmol),1-bromo-2-butyne (1.596 g, 12 mmol),Sodium carbonate (1.590 g, 15 mmol),20mL of acetone was then added to a 100mL flask, the reaction system was heated to 40 ° C, and the reaction was stirred for 4h.After the reaction was cooled to room temperature, suction filtration was performed, and the filter cake was washed with methanol to obtain a crude product as a pale yellow solid.The crude product was reconstituted with dichloromethane and cyclohexane.Yield 2.912 g of compound IV,The yield was 98% and the purity was 99.9%.
References:
CN110563728,2019,A Location in patent:Paragraph 0052-0058

1076-22-8
455 suppliers
$15.00/5g

666816-98-4
391 suppliers
$19.00/1g
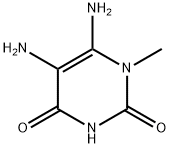
6972-82-3
128 suppliers
$21.00/100mg

666816-98-4
391 suppliers
$19.00/1g

2434-53-9
239 suppliers
$6.00/5g

666816-98-4
391 suppliers
$19.00/1g

6972-78-7
97 suppliers
$42.00/5g

666816-98-4
391 suppliers
$19.00/1g