
Methyl ((S)-1-((S)-2-(4-(4-broMophenyl)-1H-iMidazol-2-yl)pyrrolidin-1-yl)-3-Methyl-1-oxobutan-2-yl)carbaMate synthesis
- Product Name:Methyl ((S)-1-((S)-2-(4-(4-broMophenyl)-1H-iMidazol-2-yl)pyrrolidin-1-yl)-3-Methyl-1-oxobutan-2-yl)carbaMate
- CAS Number:1228552-27-9
- Molecular formula:C20H25BrN4O3
- Molecular Weight:449.34
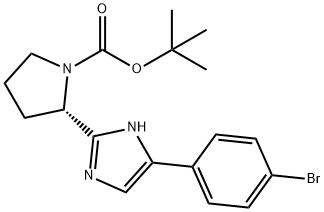
1007882-04-3
59 suppliers
inquiry
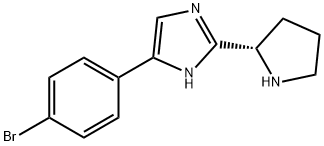
1255936-24-3
8 suppliers
inquiry

1228552-27-9
13 suppliers
inquiry
Yield:1228552-27-9 17 g
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate in dichloromethane at 20;
Steps:
1.e Step e) methyl ((S)-1-((S)-2-(5-(4-bromophenyl)-1 H-imidazol-2-yl)pyrrolidin-1-yl)-3- methyl-1-oxobutan-2-yl)carbamate (BB1-e)
A solution of Boc-protected amine BB1-d (20 g, 51.2 mmol) in DCM and TFA 1 : 1 (100 ml) was stirred at r.t. for 2h whereafter the solvents were removed under vacuum. The afforded residue was dissolved in DCM (250 ml) and the acid BB1-a (9 g, 51.2 mmol), HATU (25.3 g, 66.6 mmol) and DIPEA (19.8 g, 153.6 mmol) were added and the mixture was stirred at room temperature overnight. Most of the volatile component was removed in vacuo, and the resulting residue was partitioned between EtOAc and water. The organic layer was washed with water and brine, dried, filtered and concentrated in vacuo. The residue was purified by column (p. ether: EtOAc 3:2-1 : 1) which gave the title compound as a white solid (17 g, 74%). MS (ESI): 449 [M+H]+.
References:
WO2013/95275,2013,A1 Location in patent:Page/Page column 34
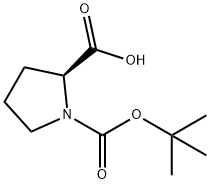
15761-39-4
602 suppliers
$5.00/10g

1228552-27-9
13 suppliers
inquiry
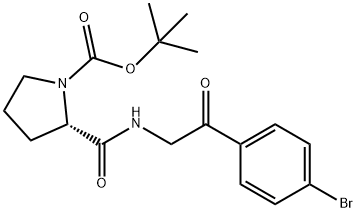
1007881-98-2
37 suppliers
inquiry

1228552-27-9
13 suppliers
inquiry

1007882-04-3
59 suppliers
inquiry

1228552-27-9
13 suppliers
inquiry
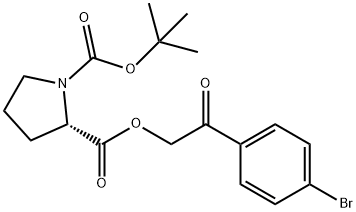
128120-93-4
1 suppliers
inquiry

1228552-27-9
13 suppliers
inquiry