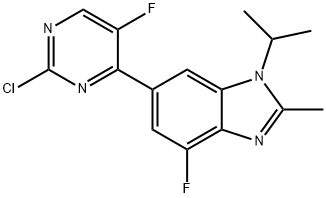
1H-BenziMidazole, 6-(2-chloro-5-fluoro-4-pyriMidinyl)-4-fluoro-2-Methyl-1-(1-Methylethyl)- synthesis
- Product Name:1H-BenziMidazole, 6-(2-chloro-5-fluoro-4-pyriMidinyl)-4-fluoro-2-Methyl-1-(1-Methylethyl)-
- CAS Number:1231930-42-9
- Molecular formula:C15H13ClF2N4
- Molecular Weight:322.74
![6-Bromo-4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole](/CAS/GIF/1231930-33-8.gif)
1231930-33-8
203 suppliers
$13.00/100mg
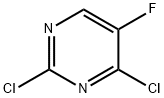
2927-71-1
413 suppliers
$6.00/5g

1231930-42-9
140 suppliers
$12.00/100mg
Yield:1231930-42-9 76%
Reaction Conditions:
Stage #1:6-bromo-4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole with isopropylmagnesium chloride in tetrahydrofuran at 0 - 5;Inert atmosphere;
Stage #2:2,4-dichloro-5-fluoropyrimidine with iron(III)-acetylacetonate in tetrahydrofuran at 55 - 60;Inert atmosphere;
Steps:
3 Example 3
Compound 3 (27.11 g, 100 mmol) and tetrahydrofuran (136 mL) were added to a three-neck flask. After stirring and dissolving, the ice salt bath is cooled to 0 to 5 ° C. Vacuum switching nitrogen 3 times, 2.0 M isopropylmagnesium chloride tetrahydrofuran solution (110 mmol, 55.0 mL) was added dropwise. The internal temperature is maintained at 0 to 5 ° C for 0.5 to 1 hour. 2,4-Dichloro-5-fluoropyrimidine (18.37 g, 110 mmol) Dissolved in tetrahydrofuran (136 mL) under nitrogen. The catalyst iron triacetylacetonate (1.78 g, 5 mmol) was added. After stirring uniformly, the prepared Grignard reagent solution is added dropwise to the reaction bottle containing pyrimidine. After the completion of the dropwise addition, the temperature was raised to 55 to 60 ° C for 4 to 6 hours. After the reaction is completed, the reaction is quenched by adding a saturated aqueous solution of ammonium chloride. The mixture was extracted three times with ethyl acetate (216 mL). The combined organic phases were washed twice (216 mL), Dry with sodium sulfate, filter, Most of the ethyl acetate was removed by concentration and petroleum ether (125 mL) was added. Precipitating solid beating, Filtered and dried, Compound 5 was obtained (24.53 g, 76%).
References:
Hangzhou Kechao Biological Technology Co., Ltd.;Zheng Xuchun;Zhang Yiping;Wu Yihua CN109761959, 2019, A Location in patent:Paragraph 0053-0055
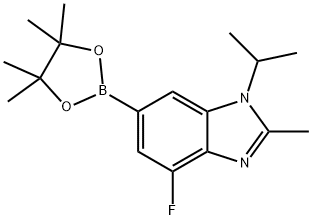
1231930-37-2
126 suppliers
$20.00/100mg

2927-71-1
413 suppliers
$6.00/5g

1231930-42-9
140 suppliers
$12.00/100mg
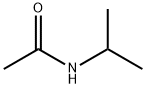
1118-69-0
92 suppliers
$59.00/25g

1231930-42-9
140 suppliers
$12.00/100mg
![6-Bromo-4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole](/CAS/GIF/1231930-33-8.gif)
1231930-33-8
203 suppliers
$13.00/100mg

1231930-42-9
140 suppliers
$12.00/100mg
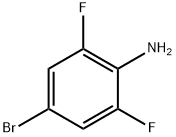
67567-26-4
339 suppliers
$5.00/1g

1231930-42-9
140 suppliers
$12.00/100mg