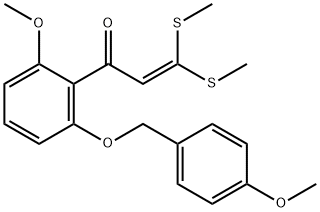
2-Propen-1-one, 1-[2-methoxy-6-[(4-methoxyphenyl)methoxy]phenyl]-3,3-bis(methylthio)- synthesis
- Product Name:2-Propen-1-one, 1-[2-methoxy-6-[(4-methoxyphenyl)methoxy]phenyl]-3,3-bis(methylthio)-
- CAS Number:1234015-62-3
- Molecular formula:C20H22O4S2
- Molecular Weight:390.52

75-15-0
238 suppliers
$40.00/100g
1234015-61-2
45 suppliers
$45.00/10mg

74-88-4
340 suppliers
$15.00/10g
![2-Propen-1-one, 1-[2-methoxy-6-[(4-methoxyphenyl)methoxy]phenyl]-3,3-bis(methylthio)-](/CAS/20180703/GIF/1234015-62-3.gif)
1234015-62-3
7 suppliers
inquiry
Yield:1234015-62-3 77%
Reaction Conditions:
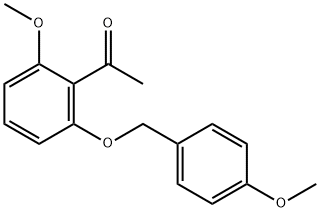
Stage #1: 1-[2-methoxy-6-(4-methoxybenzyloxy)phenyl]ethanonewith lithium tert-butoxide in dimethyl sulfoxide at 20 - 30;Inert atmosphere;
Stage #2: carbon disulfide;methyl iodide in dimethyl sulfoxide at 20 - 30;Product distribution / selectivity;Inert atmosphere;
Steps:
14
Preparation 14; 1 -(2-Methoxy-6-(4-methoxybenzyloxy)phenyl)-3 ,3 -bis(methylthio)prop-2-en- 1 -one; To a mixture of lithium tert-butoxide (602.4 g, 7.52 mol) in anhydrous DMSO (11.0 L) under a nitrogen atmosphere is added l-(2-methoxy-6-(4- methoxybenzyloxy)phenyl)ethanone (1000.0 g, 3.49 mol). The resulting mixture is stirred 30 min and CS2 (259 mL, 4.296 mol) is slowly added over 1 to 1.5 h while maintaining the internal temperature below 30 0C. After stirring for at least one hour at ambient temperature, iodomethane (1000 g, 7.045 mol) is added slowly while maintaining the internal temperature below 30 0C. The resulting mixture is stirred at ambient temperature for 30 min to one hour. Reaction completion is confirmed by HPLC. The resulting reaction mixture is cooled, followed by extractive work up with water and ethyl acetate. The resulting organic portion is concentrated to provide a slurry which is filtered and washed with ethyl acetate (1 L), followed by methyl ?-butyl ether (2 x 1 L). The isolated solid is dried at 40 0C in a vacuum oven to provide 1057 g (77%) of the title compound, mp 93 - 94 0C; ES/MS m/z 391.2 [M+ 1]+.
References:
WO2010/77758,2010,A1 Location in patent:Page/Page column 31