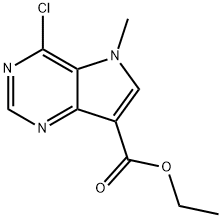
ethyl 4-chloro-5-methyl-5h-pyrrolo[3,2-d]pyrimidine-7-carboxylate synthesis
- Product Name:ethyl 4-chloro-5-methyl-5h-pyrrolo[3,2-d]pyrimidine-7-carboxylate
- CAS Number:1234616-53-5
- Molecular formula:C10H10ClN3O2
- Molecular Weight:239.66
![ETHYL 4-CHLORO-5H-PYRROLO[3,2-D]PYRIMIDINE-7-CARBOXYLATE](/CAS/GIF/853058-42-1.gif)
853058-42-1

74-88-4
![ethyl 4-chloro-5-methyl-5h-pyrrolo[3,2-d]pyrimidine-7-carboxylate](/CAS/GIF/1234616-53-5.gif)
1234616-53-5
Ethyl 4-chloro-5H-pyrrolo[3,2-d]pyrimidine-7-carboxylate (500 mg, 2.22 mmol) was dissolved in N,N-dimethylformamide (4 mL, 50 mmol) and the solution was cooled to 0 °C. Sodium hydride (60% dispersed in mineral oil, 115.2 mg) was added to the solution at 0 °C and the reaction mixture was stirred for 20 min. Subsequently, iodomethane (165.5 μL, 2.659 mmol) was added and the reaction mixture was slowly warmed to room temperature. Upon completion of the reaction, the reaction was quenched with aqueous ammonium chloride and extracted three times with dichloromethane. The organic phases were combined, washed with saturated saline, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by fast column chromatography (elution gradient: 0-100% heptane solution of ethyl acetate containing 15% methanol) to afford ethyl 4-chloro-5-methyl-5H-pyrrolo[3,2-d]pyrimidine-7-carboxylate as a solid (380 mg in 72% yield).1H NMR (500 MHz, DMSO-d6) δ 8.77 (s, 1H) 8.65 (s, 1H), 4.30 (q, J = 7.1 Hz, 2H), 4.16 (s, 3H), 1.32 (t, J = 7.1 Hz, 3H).
![ETHYL 4-CHLORO-5H-PYRROLO[3,2-D]PYRIMIDINE-7-CARBOXYLATE](/CAS/GIF/853058-42-1.gif)
853058-42-1
46 suppliers
$60.00/5g

74-88-4
357 suppliers
$15.00/10g
![ethyl 4-chloro-5-methyl-5h-pyrrolo[3,2-d]pyrimidine-7-carboxylate](/CAS/GIF/1234616-53-5.gif)
1234616-53-5
22 suppliers
$45.00/10mg
Yield:1234616-53-5 72%
Reaction Conditions:
Stage #1: 4-chloro-5H-pyrrolo[3,2-d]pyrimidine-7-carboxylic acid ethyl esterwith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.333333 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide;mineral oil at 0 - 20;
Steps:
AU.D
Ethyl 4-chloro-5H-pyrrolo[3,2-J]pyrimidine-7-carboxylate (500 mg, 2.22 mmol) was dissolved in TV^V-dimethylformamide (4 mL, 50 mmol) and cooled to 0 °C . Sodium hydride (60% in mineral oil, 115.2 mg) was added, and the mixture was stirred at 0°C for 20 minutes. Methyl iodide (165.5 uL, 2.659 mmol) was added, and the mixture was slowly warmed to room temperature. The mixture was quenched with ammonium chloride solution and extracted with dichloromethane three times. The combined extracts were washed with brine, dried over sodium sulfate and concentrated. The crude product was purified using flash chromatography (gradient elution: 0-100% ethyl acetate + 15% MeOH in heptanes) to yield ethyl 4-chloro-5-methyl-5H-pyrrolo[3,2-J]pyrimidine-7-carboxylate as a solid (380 mg, 72%). 1H NMR (500 MHz, DMSO-J6) δ 8.77 (s, IH), 8.65 (s, IH), 4.30 (q, J= 7.1 Hz, 2H), 4.16 (s, 3H), 1.32 (t, J= 7.1 Hz, 3H).
References:
WO2011/25940,2011,A1 Location in patent:Page/Page column 85-86