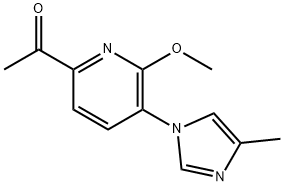
Ethanone, 1-[6-methoxy-5-(4-methyl-1H-imidazol-1-yl)-2-pyridinyl]- synthesis
- Product Name:Ethanone, 1-[6-methoxy-5-(4-methyl-1H-imidazol-1-yl)-2-pyridinyl]-
- CAS Number:1242313-72-9
- Molecular formula:C12H13N3O2
- Molecular Weight:231.25
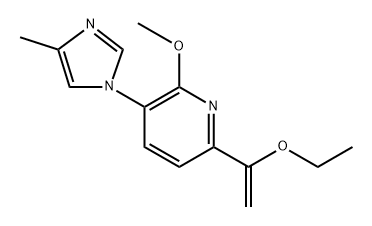
1241428-42-1
1 suppliers
inquiry
![Ethanone, 1-[6-methoxy-5-(4-methyl-1H-imidazol-1-yl)-2-pyridinyl]-](/CAS/20200611/GIF/1242313-72-9.gif)
1242313-72-9
12 suppliers
inquiry
Yield:1242313-72-9 98%
Reaction Conditions:
with hydrogenchloride;water in acetone at 25; for 18 h;
Steps:
5.7.5. 1-(6-methoxy-5-(4-methyl-1H-imidazol-1-yl)pyridin-2-yl)ethan-1-one (16a)
To a solution of 6-bromo-2-methoxy-3-(4-methyl-1H-imidazol-1-yl)pyridine (15a, 100 g, 0.373 mol), 1-(vinyloxy)butane (74.6 g,0.746 mmol), and triethylamine (75.3 g, 0.746 mol) in ethylene glycol(1000 mL) at room temperature under nitrogen was added dichloro[1,1′-bis(diphenylphosphino) ferrocene] palladium (II) dichloromethaneadduct (8.18 g, 11.2 mmol). The mixture was heated to100 °C for 4 h (reaction progress was monitored by LCMS; heating thereaction mixture for too long will result in a lower yield) and thencooled to room temperature. The mixture was diluted with 2:1 ethylacetate/water (3 L) and the resulting layers were separated. The organiclayer was washed with brine (2 × 500 mL), dried with sodiumsulfate, filtered, and concentrated. The resulting reaction residue waspurified by flash column chromatography on silica gel, eluting withheptane/ethyl acetate (gradient of 4:1 to 1:1) to afford 6-(1-butoxyvinyl)-2-methoxy-3-(4-methyl-1H-imidazol-1-yl)pyridine as a yellowsolid (78.4 g, 73%) that was suitable for use without further purification.Then aqueous hydrochloric acid (2 N, 550 mL) was added to asolution of 6-(1-butoxyvinyl)-2-methoxy-3-(4-methyl-1H-imidazol-1-yl)-pyridine (A6, 78.4 g, 0.273 mol) in acetone (2200 mL) at room temperature.After stirring the reaction mixture for 30 min, the organicsolvent was removed under reduced pressure, and the mixture wasdiluted with water (1000 mL) and extracted with ethyl acetate(2000 mL). The organic layer was washed with water (~500 mL), andthe ethyl acetate layer was discarded. With fast stirring, potassiumcarbonate was slowly added (neutralized to pH 8) to the combinedaqueous layers. The resulting mixture was extracted with ethyl acetate(2 × 2000 mL). The combined organic layer was washed with brine(2 × 500 mL) and dried over anhydrous sodium sulfate, filtered, andconcentrated to afford 1-(6-methoxy-5-(4-methyl-1H-imidazol-1-yl)-pyridin-2-yl)ethanone (16a) as a light yellow solid (60.6 g, 96%) thatwas suitable for use without further purification: LCMS (M + H) 232;1H NMR (300 MHz, CDCl3) δ 7.91 (d, J = 1.5 Hz, 1H), 7.76 (d,J = 8.0 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.04 (m, 1H), 4.18 (s, 3H),2.71 (s, 3H), 2.31 (d, J = 0.9 Hz, 3H).O NNNOBr
References:
Barnes, Keith D.;Buckle, Ronald N.;Chen, Xinchao;Herr, R. Jason;Johnson, Graham;Lin, Juinn H.;Mayhew, Nicholas J.;Mobley, William C.;Nguyen, Phuong;Paquette, William D.;Rynearson, Kevin D.;Sakwa, Samuel A.;Tanzi, Rudolph E.;Wagner, Steven L.;Yang, Jinhai [Bioorganic and Medicinal Chemistry,2020,vol. 28,# 22,art. no. 115734] Location in patent:supporting information
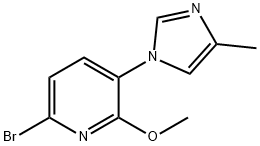
1123194-98-8
57 suppliers
$38.00/100mg
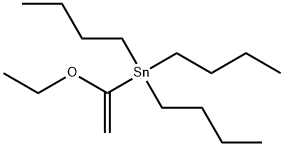
97674-02-7
232 suppliers
$15.00/1g
![Ethanone, 1-[6-methoxy-5-(4-methyl-1H-imidazol-1-yl)-2-pyridinyl]-](/CAS/20200611/GIF/1242313-72-9.gif)
1242313-72-9
12 suppliers
inquiry

1123194-98-8
57 suppliers
$38.00/100mg
![Ethanone, 1-[6-methoxy-5-(4-methyl-1H-imidazol-1-yl)-2-pyridinyl]-](/CAS/20200611/GIF/1242313-72-9.gif)
1242313-72-9
12 suppliers
inquiry
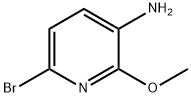
89466-18-2
177 suppliers
inquiry
![Ethanone, 1-[6-methoxy-5-(4-methyl-1H-imidazol-1-yl)-2-pyridinyl]-](/CAS/20200611/GIF/1242313-72-9.gif)
1242313-72-9
12 suppliers
inquiry

1123194-96-6
18 suppliers
$160.40/100mg
![Ethanone, 1-[6-methoxy-5-(4-methyl-1H-imidazol-1-yl)-2-pyridinyl]-](/CAS/20200611/GIF/1242313-72-9.gif)
1242313-72-9
12 suppliers
inquiry