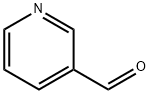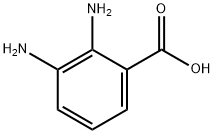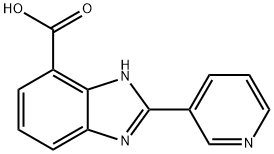
1H-Benzimidazole-7-carboxylic acid, 2-(3-pyridinyl)- synthesis
- Product Name:1H-Benzimidazole-7-carboxylic acid, 2-(3-pyridinyl)-
- CAS Number:124340-89-2
- Molecular formula:C13H9N3O2
- Molecular Weight:239.23
Yield:124340-89-2 83%
Reaction Conditions:
in N,N-dimethyl-formamide at 80; for 120 h;
Steps:
5.1.1. General procedure A: synthesis of 2-substituent-1H-benzimidazole-4-carboxylic acids (compounds 7-13)
General procedure: Appropriate aldehyde (10.5 mmol) was added to the solution of 2,3-diaminobenzoic acid 6 (1.52 g, 10 mmol) in DMF (15 mL). The solution was heated to 80 °C and then stirred for about 120 h. Then, the solution was cooled to room temperature. The precipitates were filtered, washed with ethanol and dried.
References:
Xue, Fei;Luo, Xianjin;Ye, Chenghao;Ye, Weidong;Wang, Yue [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 8,p. 2641 - 2649] Location in patent:experimental part
606-18-8
296 suppliers
$9.00/1g

124340-89-2
6 suppliers
inquiry
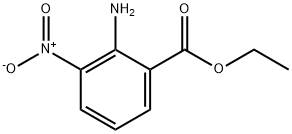
61063-11-4
28 suppliers
inquiry

124340-89-2
6 suppliers
inquiry
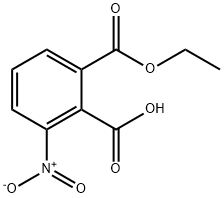
16533-45-2
95 suppliers
$7.00/250mg

124340-89-2
6 suppliers
inquiry

136285-66-0
2 suppliers
inquiry

124340-89-2
6 suppliers
inquiry