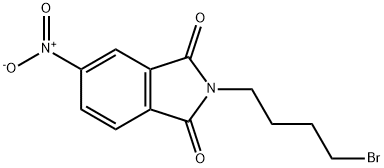
2-(4-BROMOBUTYL)-5-NITRO-1H-ISOINDOLE-1,3(2H)-DIONE synthesis
- Product Name:2-(4-BROMOBUTYL)-5-NITRO-1H-ISOINDOLE-1,3(2H)-DIONE
- CAS Number:125207-39-8
- Molecular formula:C12H11BrN2O4
- Molecular Weight:327.13
Yield:-
Reaction Conditions:
in N-methyl-acetamide;acetone;
Steps:
R.1 2-(4-Bromobutyl)-5-nitro-1H-isoindole-1,3(2H)-dione STR14
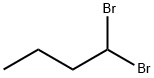
REFERENCE EXAMPLE 1 2-(4-Bromobutyl)-5-nitro-1H-isoindole-1,3(2H)-dione STR14 To a solution of 9.6 g of 4-nitrophthalimide in 50 ml of dimethylformamide was added slowly 1.26 g of sodium hydride, and the mixture was stirred at 60° C. for 30 minutes. A solution of 22 g of dibromobutane in 50 ml of acetone was added to the reaction mixture, and the whole mixture was heated under reflux for 16 hours. The mixture was then allowed to cool, the precipitate was removed, the solvents were distilled off under reduced pressure, and the residual solid was recrystallized from dichloromethane-ether (1:10, v/v) to give 14.7 g of white crystals having a melting point of 95°-96° C. Elemental analysis Calculated for C12 H11 BrN2 O4: C, 44.06; H, 3.39; N, 8.56; Found: C, 44.01; H, 3.20; N, 8.42.
References:
US5025033,1991,A
85-41-6
470 suppliers
$9.00/1g

125207-39-8
19 suppliers
$65.00/50mg