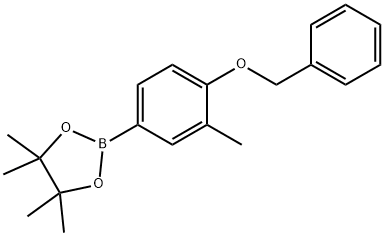
2-(4-(benzyloxy)-3-methylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane synthesis
- Product Name:2-(4-(benzyloxy)-3-methylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS Number:1257078-80-0
- Molecular formula:C20H25BO3
- Molecular Weight:324.22
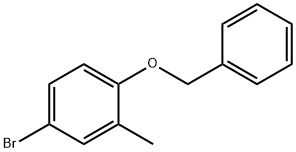
338454-32-3
22 suppliers
$120.00/100mg

73183-34-3
569 suppliers
$6.00/5g

1257078-80-0
21 suppliers
inquiry
Yield:1257078-80-0 5.16 g
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in dimethyl sulfoxide at 80; for 6 h;Inert atmosphere;
Steps:
71 Reference Production Example 71
Reference Production Example 71 (1442) A mixture of 7.40 g of CA70 mentioned in Reference Production Example 70, 8.14 g of bis(pinacolato)diboron, 1.33 g of [1,1′-bis(diphenylphosphino)ferrocene]palladium(II) dichloride dichloromethane adduct, 7.86 g of potassium acetate, and 50 mL of dimethyl sulfoxide was stirred in a nitrogen atmosphere at 80° C. for 6 hours while heating. Water was poured into the reaction mixture allowed to cool and the mixture was extracted with ethyl acetate. The organic layer was washed with water and a saturated saline solution, dried over anhydrous magnesium sulfate, and then concentrated under reduced pressure. The residue thus obtained was subjected to silica gel column chromatography to obtain 5.16 g of 4-benzyloxy-3-methyl-phenylboronic acid pinacol ester (referred to as CA71). (1443) 1H-NMR (CDCl3) δ: 1.34 (12H, s), 2.29 (3H, s), 5.12 (2H, s), 6.89 (1H, d, J=8.7 Hz), 7.33 (1H, d, J=7.1 Hz), 7.38 (2H, t, J=7.5 Hz), 7.46-7.43 (2H, m), 7.61-7.65 (2H, m).
References:
US2016/150787,2016,A1 Location in patent:Paragraph 1442-1443

2362-12-1
187 suppliers
$5.00/1g

100-39-0
429 suppliers
$10.00/10g

1257078-80-0
21 suppliers
inquiry