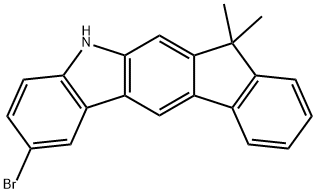
2-bromo-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole synthesis
- Product Name:2-bromo-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole
- CAS Number:1257247-97-4
- Molecular formula:C21H16BrN
- Molecular Weight:362.26
![5,7-Dihydro-7,7-dimethyl-indeno[2,1-b]carbazole](/CAS/GIF/1257220-47-5.gif)
1257220-47-5
132 suppliers
$23.00/250mg
![2-bromo-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole](/CAS/20180629/GIF/1257247-97-4.gif)
1257247-97-4
3 suppliers
inquiry
Yield:1257247-97-4 97%
Reaction Conditions:
with NBS in acetonitrile; for 1 h;Inert atmosphere;Cooling with ice;
Steps:
Intermediate Synthesis 20: Synthesis of Intermediate 20
Under an argon atmosphere, into a mixture of 5.60 g (19.8 mmol) of the intermediate 19 in 99 mL of acetonitrile, 3.52 g (19.8 mmol) of N-bromosuccinimide was added under ice-cooling. The resultant mixture was stirred for one hour. (0262) After the reaction, the mixture was heated to room temperature and the generated solid was collected by filtration. The filtrate was extracted with ethyl acetate, washed with water, dried over anhydrous magnesium sulfate, filtered, and then concentrated. The concentrate residue and the solid collected by filtration was combined and purified by silica gel column chromatography to obtain 6.93 g (yield: 97%) of solid, which was identified as the following intermediate 20 by FD-MS analysis.
References:
US2018/29983,2018,A1 Location in patent:Paragraph 0261-0262
28320-31-2
310 suppliers
$5.00/1g
![2-bromo-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole](/CAS/20180629/GIF/1257247-97-4.gif)
1257247-97-4
3 suppliers
inquiry

62-53-3
678 suppliers
$10.00/1g
![2-bromo-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole](/CAS/20180629/GIF/1257247-97-4.gif)
1257247-97-4
3 suppliers
inquiry

355832-04-1
137 suppliers
$8.00/250mg
![2-bromo-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole](/CAS/20180629/GIF/1257247-97-4.gif)
1257247-97-4
3 suppliers
inquiry

577-19-5
16 suppliers
$14.00/5g
![2-bromo-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole](/CAS/20180629/GIF/1257247-97-4.gif)
1257247-97-4
3 suppliers
inquiry