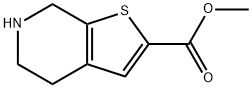
Thieno[2,3-c]pyridine-2-carboxylic acid, 4,5,6,7-tetrahydro-, Methyl ester synthesis
- Product Name:Thieno[2,3-c]pyridine-2-carboxylic acid, 4,5,6,7-tetrahydro-, Methyl ester
- CAS Number:1259287-72-3
- Molecular formula:C9H11NO2S
- Molecular Weight:197.25
![Methyl 6-Methyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-2-carboxyIate](/CAS/GIF/721926-88-1.gif)
721926-88-1
4 suppliers
inquiry
![Thieno[2,3-c]pyridine-2-carboxylic acid, 4,5,6,7-tetrahydro-, Methyl ester](/CAS/GIF/1259287-72-3.gif)
1259287-72-3
7 suppliers
inquiry
Yield:1259287-72-3 55%
Reaction Conditions:
Stage #1: methyl 6-methyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-2-carboxylatewith carbonochloridic acid 1-chloro-ethyl ester;N-ethyl-N,N-diisopropylamine in 1,4-dioxane at 0 - 20; for 3 h;
Stage #2: with methanol for 3 h;Reflux;
Steps:
1.3
Into a 1000-mL round-bottom flask containing methyl 6-methyl-4,5,6,7-tetrahydrothieno[2,3 c]pyridine-2-carboxylate (9.84 g, 46.6 mmol), and 1,4-dioxane (466 mL) was added N1N- diisopropylethylamine (81.1 mL, 466 mmol). The solution was cooled to 0 0C whereupon α-chloroethyl chloroformate (50.2 mL, 466 mmol) was added dropwise. Upon complete addition, the solution was warmed to room temperature and stirred for 3 h. The solvent was removed in vacuo and the residue obtained was dissolved in methanol (466 mL). The clear yellow solution heated to reflux for 3 h. Removal of the solvent under reduced pressure afforded a solid which was partitioned between dichloromethane and saturated aqueous NaHCO3. The aqueous layer was further extracted with dichloromethane three times. The combined organic phases were washed with brine, dried over anhydrous MgSO4 and concentrated. Purification via flash chromatography (50% EtOAc-hexanes to 1% triethylamine-99% EtOAc to 1% MeOH- 1% triethylamine-98% EtOAc) afforded methyl 4,5,6,7- tetrahydrothieno[2,3-c]pyridine-2-carboxylate (5.08 g, 55%) as a brown solid. LCMS: (FA) ES+ 198; 1H NMR (CDCl3, 300 MHz) δ 7.50 (s, 1 H)5 4.04 (s, 2 H), 3.86 (s, 3 H), 3.12 (/, J = 6.0 Hz, 2 H), 2.66 (/, J = 6.0 Hz, 2 H).
References:
WO2010/151317,2010,A1 Location in patent:Page/Page column 98; 99; 100
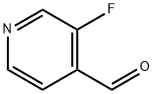
40273-47-0
146 suppliers
$9.00/1g
![Thieno[2,3-c]pyridine-2-carboxylic acid, 4,5,6,7-tetrahydro-, Methyl ester](/CAS/GIF/1259287-72-3.gif)
1259287-72-3
7 suppliers
inquiry