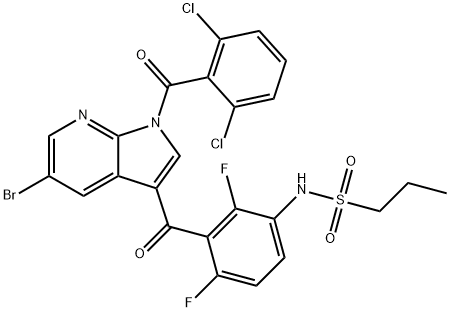
1-PropanesulfonaMide, N-[3-[[5-broMo-1-(2,6-dichlorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]- synthesis
- Product Name:1-PropanesulfonaMide, N-[3-[[5-broMo-1-(2,6-dichlorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]-
- CAS Number:1262985-24-9
- Molecular formula:C24H16BrCl2F2N3O4S
- Molecular Weight:631.27
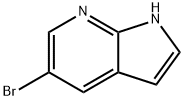
![1-PropanesulfonaMide, N-[3-[(5-broMo-1H-pyrrolo[2,3-b]pyridin-3-yl)carbonyl]-2,4-difluorophenyl]-](/CAS/GIF/918504-27-5.gif)
918504-27-5
63 suppliers
$68.00/100mg
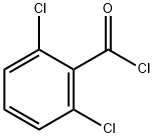
4659-45-4
269 suppliers
$12.00/5g
![1-PropanesulfonaMide, N-[3-[[5-broMo-1-(2,6-dichlorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]-](/CAS/GIF/1262985-24-9.gif)
1262985-24-9
18 suppliers
$419.00/100mg
Yield: 90%
Reaction Conditions:
with tri-n-propylamine;dmap in toluene at 20 - 25;
Steps:
2.b
Example 2; Step b; Formation of Compound (5); 45.8 g of the compound (4) as obtained according to Example 1 were suspended in600 ml toluene. Water as contained in the suspension was removed at a temperature between 60-80° C. and under reduced pressure of 450-400 mbar. Subsequently,200 ml toluene were newly added and the suspension was cooled to 20-25° C. Then, a solution of1.22 g dimethylaminopyridine in20 ml toluene was added, prior to the addition of15.8 g n-tripropylamine. Subsequently,22.0 g 2,6-dichlorobenzoylchloride were slowly added via a dropping funnel over approximately 15 minutes while the mixture was kept between 20 and 25° C.The reaction mixture was stirred for about 1-2 hours at a temperature between 20-25° C., whereby the color of the mixture turned into brown.The brownish reaction mixture as obtained by the last step above, was diluted with275 ml water and subsequently with29.6 g hydrochloric acid (37%). The resulting two phase mixture was heated to 65-70° C. The two phases were allowed to separate after about 10 minutes. The toluene phase was washed at a temperature between 65 and 70° C., first with300 ml of an aqueous solution containing 10% sodium hydrogencarbonate, and then with300 ml water. The organic (toluene) phase was concentrated by evaporation at temperatures between 55 and 60° C. and at reduced pressure (200-80 mbar) to a volume of about 200 ml. During this procedure the crude product (5) precipitated due to crystallization. The resulting suspension was then slowly cooled down (within about 5 h) to -5 to 0° C. and further stirred at that temperature for 1 h. The crude product was separated by filtration, washed twice with30 ml toluene (0° C.), and was subsequently dried at 50-55° C. and 26-13 mbar.Yield: 57 g (90%) of compound of formula (5).
References:
Hildbrand, Stefan;Mair, Hans-Juergen;Radinov, Roumen Nikolaev;Ren, Yi;Wright, James Anderson US2011/28511, 2011, A1 Location in patent:Page/Page column 9
183208-35-7
540 suppliers
$5.00/1g
![1-PropanesulfonaMide, N-[3-[[5-broMo-1-(2,6-dichlorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]-](/CAS/GIF/1262985-24-9.gif)
1262985-24-9
18 suppliers
$419.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![1-PropanesulfonaMide, N-[3-[[5-broMo-1-(2,6-dichlorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]-](/CAS/GIF/1262985-24-9.gif)
1262985-24-9
18 suppliers
$419.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![1-PropanesulfonaMide, N-[3-[[5-broMo-1-(2,6-dichlorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]-](/CAS/GIF/1262985-24-9.gif)
1262985-24-9
18 suppliers
$419.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![1-PropanesulfonaMide, N-[3-[[5-broMo-1-(2,6-dichlorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]-](/CAS/GIF/1262985-24-9.gif)
1262985-24-9
18 suppliers
$419.00/100mg