
1-Isopropyl-1H-pyrazole-5-boronic acid, pinacol ester synthesis
- Product Name:1-Isopropyl-1H-pyrazole-5-boronic acid, pinacol ester
- CAS Number:1282518-60-8
- Molecular formula:C12H21BN2O2
- Molecular Weight:236.12

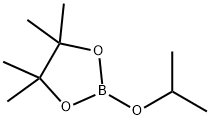
61676-62-8
313 suppliers
$11.19/5G
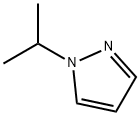
18952-87-9
142 suppliers
$18.00/1g

1282518-60-8
114 suppliers
$43.00/1g
Yield:1282518-60-8 77%
Reaction Conditions:
Stage #1: 1-isopropyl-1H-pyrazolewith n-butyllithium in tetrahydrofuran at -60 - -45; for 2 h;Inert atmosphere;Large scale;
Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran at -35;Large scale;
Steps:
2-1; 2-2 Step 2-1
Add 8500mL of tetrahydrofuran to the 20L reaction flask, add 1057g of compound 3 (9.6mol, 1.0eq) prepared in Example 1-1 under stirring, replace nitrogen, and cool the reaction system to -60°C , 4.2L of 2.5M n-butyllithium (10.5mol, 1.09eq) was added dropwise. During the dropwise addition, the temperature of the reaction system was controlled not to exceed -45°C. After the dropwise addition of n-butyllithium, the reaction was stirred for 2 hours. 1952g of compound 4 (10.5mol, 1.09eq) was added dropwise under the condition of not exceeding -35°C, the reaction was monitored by gas phase equipment, and the next step was entered after the reaction was completed;Step 2-2, add 5000 mL of saturated aqueous ammonium chloride solution to quench the reaction, heat to 40°C to evaporate tetrahydrofuran under reduced pressure, add 6000 mL of ethyl acetate, stir for 1 h, filter, wash with ethyl acetate (2000 mL×3), combine the organic phases, Heating to 35°C, ethyl acetate was distilled off under reduced pressure to obtain the concentrated crude product; 2000 mL of n-hexane was added to the concentrated crude product, heated to 40°C to dissolve the system, cooled to -20°C, stirred and crystallized for 3 hours, filtered, and cooled to 20°C. Vacuum drying to obtain 1745 g of the target compound 1-isopropylpyrazole-5-boronic acid pinacol ester with a yield of 77% and a purity of 99.3%.
References:
CN114181237,2022,A Location in patent:Paragraph 0086-0095