
1-Ethyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole synthesis
- Product Name:1-Ethyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
- CAS Number:1007110-53-3
- Molecular formula:C11H19BN2O2
- Molecular Weight:222.09
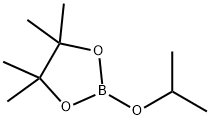
61676-62-8
313 suppliers
$10.00/5g
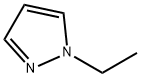
2817-71-2
155 suppliers
$5.00/1g

1007110-53-3
91 suppliers
$40.00/1g
Yield:-
Reaction Conditions:
Stage #1:1-methyl-1H-pyrazole with n-butyllithium in tetrahydrofuran;hexane at 20; for 1 h;
Stage #2:2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;hexane at -78 - 0; for 1.25 h;
Steps:
81.a
To a suspension of NaH (60% in mineral oil, 2.2 g, 55 mmol) in THF (50 ml.) was added pyrazole (3.4 g, 50 mmol) in THF (10 ml.) at room temperature. After 30 min, to above suspension was added EtI (7.75 g, 50 mmol) dropwise. After the reaction was complete (20 h), the suspension was filtered, and the resulting solution was used directly with further purification.At 00C, to above solution of 4-methyl pyrazole (-50 mmol) was added n-BuLi (2.5M in hexane, 22 ml_, 55 mmol). The reaction solution was stirred for 1 hour at RT and then cooled to -78°C [J. Heterocyclic Chem. 41 , 931 (2004)]. To the reaction solution was added 2-isopropoxy-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane (10.2 g, 55 mmol). After 15 min at -78°C, the reaction was allowed to warm to 00C over 1 hour. The reaction was diluted with saturated NH4CI solution and extracted with DCM. The organics were dried over Na2SO4 and concentrated under vacuum to afford a tan solid (9.8 g, 89%) which was used without further purification. LCMS (ES) m/z 141 (M+H)+ for [RB(OH)2]; 1H NMR (CDCI3, 400 MHz) δ ppm 7.52 (d, J =
References:
SMITHKLINE BEECHAM CORPORATION WO2008/98104, 2008, A1 Location in patent:Page/Page column 168-169