
ethyl 2-(4'-(4-(tert-butoxycarbonylamino)-3-methylisoxazol-5-yl)biphenyl-4-yl)acetate synthesis
- Product Name:ethyl 2-(4'-(4-(tert-butoxycarbonylamino)-3-methylisoxazol-5-yl)biphenyl-4-yl)acetate
- CAS Number:1284212-82-3
- Molecular formula:C25H28N2O5
- Molecular Weight:436.5002
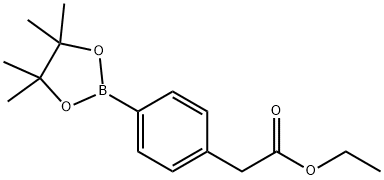
859169-20-3
106 suppliers
$17.00/100mg
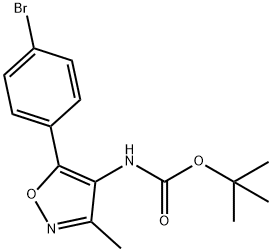
1280205-96-0
3 suppliers
inquiry

1284212-82-3
2 suppliers
inquiry
Yield:1284212-82-3 48%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;sodium carbonate in 1,4-dioxane;water;Reflux;Inert atmosphere;
Steps:
VII
A flask was charged with compound VII-2 (50 mg, 0.14 mmol), compound VII-3 (82.4 mg, 0.28 mmol), Na2C03 (30 mg, 0.28 mmol), dioxane/H20 (12 mL, v/v=5/l) and Pd(dppf)Cl2 (20 mg, 0.03 mmol). The flask was purged with nitrogen and the mixture was refluxed overnight. After being cooled to rt, the mixture was diluted with water (10 mL), extracted with EtOAc (20 mL><3). The combined organic layer was washed with brine, dried over anhydrous Na2S04, and concentrated in vacuo. The residue was purified by prep-TLC (Petroleum ether:EtOAc = 3: 1) to give compound VII-4 (30 mg, yield 48%). 1H NMR (CDC13, 400 MHz): δ 7.88 (d, J=7.2 Hz, 2H), 7.69 (d, J=6.4 Hz, 2H), 7.59 (d, J=6.4 Hz, 2H), 7.39 (d, J=8.4 Hz, 2 H), 5.8 (brs, 1H), 4.18 (q, 2H), 3.67 (s, 2H), 2.30 (s, 3H), 1.51 (brs, 9H), 1.28 (t, J=7.2 Hz, 3H). MS (ESI) m/z (M+H)+ 437.
References:
WO2013/25733,2013,A1 Location in patent:Paragraph 0454

1228689-61-9
32 suppliers
$22.00/100mg

1284212-82-3
2 suppliers
inquiry
91182-60-4
67 suppliers
$37.00/100mg

1284212-82-3
2 suppliers
inquiry
586-76-5
483 suppliers
$10.00/1g

1284212-82-3
2 suppliers
inquiry
586-75-4
217 suppliers
$10.00/5g

1284212-82-3
2 suppliers
inquiry