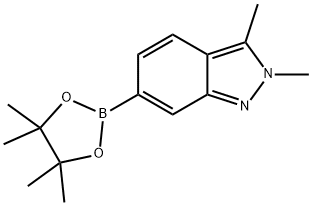
2,3-Dimethyl-2H-indazole-6-boronic acid pinacol ester synthesis
- Product Name:2,3-Dimethyl-2H-indazole-6-boronic acid pinacol ester
- CAS Number:1300582-62-0
- Molecular formula:C15H21BN2O2
- Molecular Weight:272.15

1142189-49-8
46 suppliers
$130.00/250mg

73183-34-3
563 suppliers
$6.00/5g

1300582-62-0
11 suppliers
$1065.00/100mg
Yield:1300582-62-0 83%
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,4-dioxane at 80; for 12.5 h;Inert atmosphere;
Steps:
116
Intermediate 116 2,3-dimethyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2H-indazole To a solution of intermediate 114b (0.80 g, 3.55 mmoles) in Dioxan (14 ml), bis(pinacaloto)diboron (0.992 g, 3.90 mmoles) and potassium acetate (0.697 g, 7.10 mmoles) were added and the system is degassed for 30 min. Bis(diphenylphosphinoferrocene)dichloro palladium.CH2Cl2 (0.145 g, 0.177 mmoles) was added under nitrogen atmosphere and heated to 80° C. After 12 h, the reaction mixture filtered through celite and concentrated. The crude product was purified by column chromatography with ethyl acetate:petroleum ether to afford the title compound as off-white solid (0.80 g, 83% yield).). 1H-NMR (δ ppm, DMSO-d6, 400 MHz): δ 7.85 (s, 1H), 7.62 (dd, J=8.3, 0.8 Hz, 1H), 7.19 (d, J=8.4 Hz, 1H), 4.05 (s, 3H), 2.58 (s, 3H), 1.29 (s, 12H).
References:
US2011/118257,2011,A1 Location in patent:Page/Page column 100
57848-46-1
384 suppliers
$9.00/1g

1300582-62-0
11 suppliers
$1065.00/100mg
625446-22-2
192 suppliers
$6.00/250mg

1300582-62-0
11 suppliers
$1065.00/100mg

353282-88-9
16 suppliers
inquiry

1300582-62-0
11 suppliers
$1065.00/100mg
7746-27-2
118 suppliers
$12.00/250mg

1300582-62-0
11 suppliers
$1065.00/100mg