
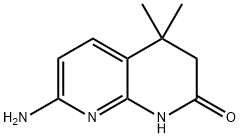
7-Chloro-4,4-dimethyl-3,4-dihydro-1,8-naphthyridin-2(1H)-one synthesis
- Product Name:7-Chloro-4,4-dimethyl-3,4-dihydro-1,8-naphthyridin-2(1H)-one
- CAS Number:1303588-33-1
- Molecular formula:C10H11ClN2O
- Molecular Weight:210.66
618446-06-3
24 suppliers
$57.00/100mg

1303588-33-1
5 suppliers
inquiry
Yield:1303588-33-1 50%
Reaction Conditions:
Stage #1: 7-amino-4,4-dimethyl-3,4-dihydro-1H-[1,8]naphthyridin-2-onewith hydrogenchloride;sodium nitrite in water; for 0.5 h;
Stage #2: with copper(l) chloride in water; for 2 h;
Stage #3: with ammonium hydroxide in water; pH=9 - 10;
Steps:
28.3
To a mixture of 7-amino-4,4-dimethyl-3,4-dihydro-l,8-naphthyridin-2(l H)-one (119; 191 mg, 1.0 mmol, 1.0 eq) in 2 mL of concentrated hydrochloric acid at 0°C was added a solution of NaN02 in water (386 mg/0.5 mL). After stirring for 30 min, powdered CuCl (150 mg, 1.5 mmol) was added to the above mixture and stirred for 2 hours. Water (5 mL) was added to the reaction mixture and the pH adjusted to -9-10 with NH4OH and then extracted with ethyl acetate (2*). The combined organic layers were washed with water, brine, and concentrated in vacuo to provide a yellow solid. The crude material was loaded onto a silica gel flash column using ethyl acetate/petroleum ether = 10:1 as eluent to afford a yellow solid of 7-chloro-4,4- dimethyl-3,4-dihydro-l,8-naphthyridin-2(lH)-one (120; 105 mg, 50%). MS (ESI) calcd for CioHuCIN20: 210.06.
References:
WO2011/59839,2011,A1 Location in patent:Page/Page column 121
3350-78-5
117 suppliers
$31.80/1gm:

1303588-33-1
5 suppliers
inquiry

141-86-6
504 suppliers
$6.00/5g

1303588-33-1
5 suppliers
inquiry