
2-AMino-4-oxo-6,7-dihydro-4H-thiazolo[5,4-c]pyridine-5-carboxylic acid tert-butyl ester synthesis
- Product Name:2-AMino-4-oxo-6,7-dihydro-4H-thiazolo[5,4-c]pyridine-5-carboxylic acid tert-butyl ester
- CAS Number:1312412-88-6
- Molecular formula:C11H15N3O3S
- Molecular Weight:269.32
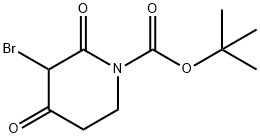
1312412-87-5
28 suppliers
$80.00/100mg

17356-08-0
3 suppliers
inquiry
![2-AMino-4-oxo-6,7-dihydro-4H-thiazolo[5,4-c]pyridine-5-carboxylic acid tert-butyl ester](/CAS/20150408/GIF/1312412-88-6.gif)
1312412-88-6
20 suppliers
inquiry
Yield:1312412-88-6 76%
Reaction Conditions:
with sodium hydrogencarbonate in ethanol at 80; for 2.5 h;
Steps:
II(a).iv; 1.iv Step iv: tert-butyl 2-amino-4-oxo-6,7-dihvdrothiazolo[5,4-clpyridine-5(4H)-carboxylate
Step iv: tert-butyl 2-amino-4-oxo-6,7-dihvdrothiazolo[5,4-clpyridine-5(4H)-carboxylate
To a 50 mL round bottom flask, were added tert-butyl 3-bromo-2,4-dioxopiperidine-l- carboxylate (1 g, 0.0034 mol), thiourea (0.287 g, 0.0038 mol), sodium bicarbonate (0.317 g, 0.0038 mol) and ethanol (15 mL). The reaction mixture was stirred at 80 °C for 2.5 h. The volatiles were evaporated under reduced pressure to get residue. The residue was partitioned between ethyl acetate and water. The organic layer was separated, washed with brine and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to obtain the title compound [0.7 g, 76 %]. NMR (300 MHz, CDsOD): δ 4.05 (t, 2H), 2.83 (t, 2H), 1.52 (s, 9H).
References:
WO2015/101928,2015,A1 Location in patent:Page/Page column 52; 54
845267-78-9
167 suppliers
$10.00/1g
![2-AMino-4-oxo-6,7-dihydro-4H-thiazolo[5,4-c]pyridine-5-carboxylic acid tert-butyl ester](/CAS/20150408/GIF/1312412-88-6.gif)
1312412-88-6
20 suppliers
inquiry
3303-84-2
362 suppliers
$6.00/5g
![2-AMino-4-oxo-6,7-dihydro-4H-thiazolo[5,4-c]pyridine-5-carboxylic acid tert-butyl ester](/CAS/20150408/GIF/1312412-88-6.gif)
1312412-88-6
20 suppliers
inquiry

24424-99-5
825 suppliers
$13.50/25G
![2-AMino-4-oxo-6,7-dihydro-4H-thiazolo[5,4-c]pyridine-5-carboxylic acid tert-butyl ester](/CAS/20150408/GIF/1312412-88-6.gif)
1312412-88-6
20 suppliers
inquiry