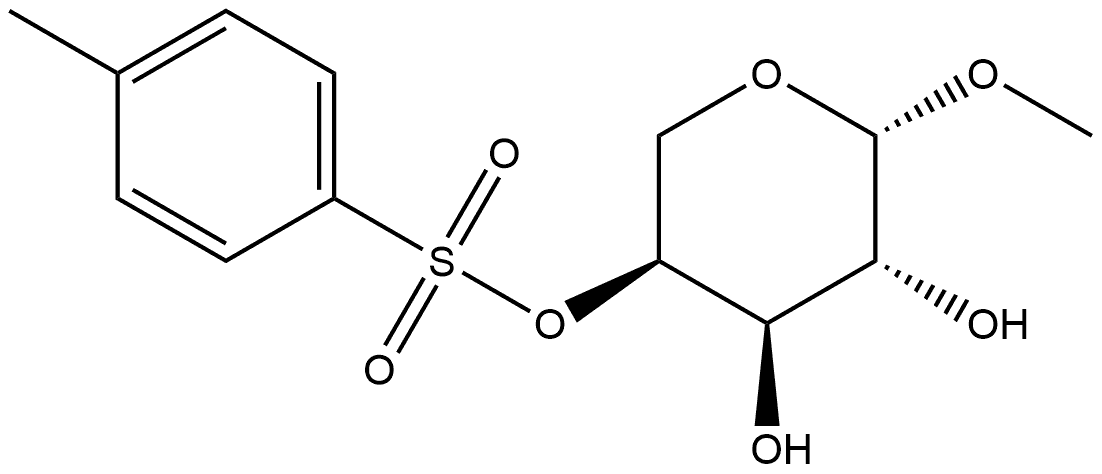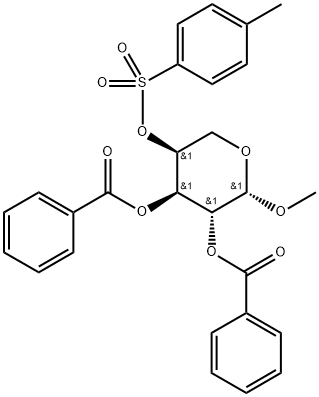
Methyl 2-O,3-O-dibenzoyl-4-O-(p-tolylsulfonyl)-β-L-arabinopyranoside synthesis
- Product Name:Methyl 2-O,3-O-dibenzoyl-4-O-(p-tolylsulfonyl)-β-L-arabinopyranoside
- CAS Number:13143-92-5
- Molecular formula:C27H26O9S
- Molecular Weight:526.56
Yield:13143-92-5 92%
Reaction Conditions:
with pyridine;dmap at 0 - 20; for 26 h;
Steps:
6.4 Methyl-2,3-di-O-benzoyl-4-O-tosyl-β-l-arabinopyranoside (6)24
To an ice cooled solution of 5 (37.4g, 117.48mmol) and DMAP (2.87g, 23.5mmol) in dry pyridine (600mL) was added dropwise a mixture of benzoyl chloride (54.6mL, 469.9mmol) in dry pyridine (100mL) over a period of 1.5h. After 30min, the ice bath was removed and the reaction mixture was stirred at room temperature for 24h. Next, the excess of benzoyl chloride was quenched by adding saturated NaHCO3 (1 L) dropwise. The solvent was evaporated to dryness and the residue was partitioned between saturated NaHCO3 (1 L) and ethyl acetate (1 L). The layers were separated and the aqueous layer was further washed with ethyl acetate (2×500mL). The organic layer was collected, dried over Na2SO4, filtered and evaporated to dryness. The crude yellow residue was subjected to column chromatography to yield 56.8g (107.87mmol, 92%) of di-O-benzoylated product 6 as a white solid. 1H NMR (600MHz, CDCl3) δ 2.17 (s, 3H, Ts-CH3), 3.42 (s, 3H, OCH3), 3.96-4.05 (m, 2H, H-5), 5.08 (bs, 1H, H-4), 5.14 (d, 1H, J=3.0Hz, H-1), 5.59 (t, 2H, J=2.4Hz, H-2 and H-3), 6.94-6.97 (m, 2H, ortho-H, Ts), 7.33-7.36 (m, 4H, Bz), 7.48-7.53 (m, 2H, Bz), 7.65-7.68 (m, 2H, meta-H, Ts), 7.75-7.78 (m, 2H, Bz), 7.93-7.95 (m, 2H, Bz); 13C NMR (150MHz, CDCl3) δ 55.8 (OCH3), 60.6 (C-5), 67.8 (C-3), 68.4 (C-2), 76.8 (C-4), 97.7 (C-1), 127.7, 128.2, 128.4, 128.8, 129.2, 129.7, 129.78, 129.80, 133.1, 133.28, 133.29, 144.7 (aromatic), 165.5, 165.7 (C=O, Bz); HRMS for C27H30O9SN [M+NH4]+calcd.: 544.1641; found: 544.1631.
References:
De Graef, Steff;Gadakh, Bharat;Nautiyal, Manesh;Pang, Luping;Strelkov, Sergei V.;Van Aerschot, Arthur;Vondenhoff, Gaston;Weeks, Stephen D. [Bioorganic and medicinal chemistry,2020,vol. 28,# 17]
5328-37-0
518 suppliers
$5.00/1g

13143-92-5
1 suppliers
inquiry