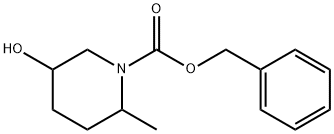
5-Hydroxy-2-Methyl-Piperidine-1-Carboxylic Acid Benzyl Ester synthesis
- Product Name:5-Hydroxy-2-Methyl-Piperidine-1-Carboxylic Acid Benzyl Ester
- CAS Number:1314396-07-0
- Molecular formula:C14H19NO3
- Molecular Weight:249.31
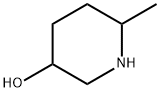
54751-93-8
10 suppliers
inquiry

501-53-1
395 suppliers
$10.00/1g

1314396-07-0
19 suppliers
$771.00/1g
Yield:1314396-07-0 42%
Reaction Conditions:
with triethylamine in dichloromethane; for 16 h;
Steps:
121.2 Step 2: preparation of benzyl 5-hydroxy-2-methylpiperidine-i-carboxylate.
Step 2: preparation of benzyl 5-hydroxy-2-methylpiperidine-i-carboxylate. To a solution of 6-methylpiperidin-3-ol (9.0 g, 78.1 mmol) in dichloromethane (100 mL) wasadded drop wise triethylamine (101 mL, 703 mmol), followed by benzyl chloroformate (14 mL, 93.8 mmol). The reaction was allowed to stir over 16h, after which it was concentrated in vacuo, and the resulting residue purified by silica gel column chromatography to afford the title compound as colorless oil (8.ig 42%).
References:
WO2014/68527,2014,A1 Location in patent:Page/Page column 150
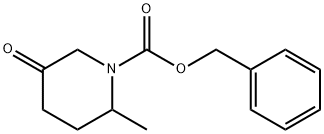
1314395-91-9
16 suppliers
$45.00/10mg

1314396-07-0
19 suppliers
$771.00/1g
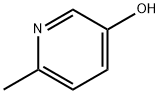
1121-78-4
289 suppliers
$6.00/5g

1314396-07-0
19 suppliers
$771.00/1g

501-53-1
395 suppliers
$10.00/1g

1314396-07-0
19 suppliers
$771.00/1g