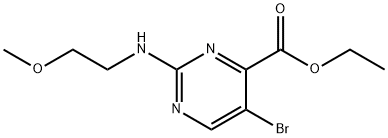
Ethyl 5-bromo-2-((2-methoxyethyl)amino)pyrimidine-4-carboxylate synthesis
- Product Name:Ethyl 5-bromo-2-((2-methoxyethyl)amino)pyrimidine-4-carboxylate
- CAS Number:1316122-39-0
- Molecular formula:C10H14BrN3O3
- Molecular Weight:304.14
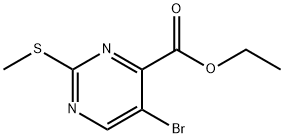
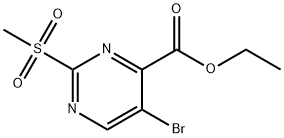
74840-38-3
28 suppliers
$34.00/250mg

109-85-3
262 suppliers
$10.00/1g

1316122-39-0
4 suppliers
inquiry
Yield:1316122-39-0 89%
Reaction Conditions:
in dichloromethane at 20 - 45; for 2 h;
Steps:
A.2
Step 2: 5-Bromo-2-(2-methoxy-ethylamino)-pyrimidine-4-carboxylic acid ethyl ester2-Methoxyethylamine (0.278 ml, 3.2 mmol) was added at room temperature to a solution of 5- bromo-2-methanesulfonyl-pyrimidine-4-carboxylic acid ethyl ester (0.2 g, 0.65 mmol) in dichloromethane (5 ml). Stirring was continued at 45 °C for 2 hours. The solvent was evaporated and the crude product was purified by silica gel chromatography using an ethyl acetate/heptane eluent to yield the title compound as colorless oil (0.175 g, 89 %).MS: M = 304.2 (M+H)+
References:
WO2011/89132,2011,A1 Location in patent:Page/Page column 55

74840-38-3
28 suppliers
$34.00/250mg

1316122-39-0
4 suppliers
inquiry