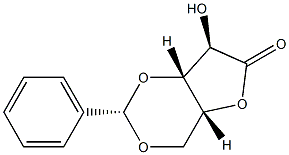
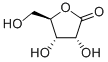
3,5-O-[(S)-phenylMethylene]-, γ-lactone D-Xylonic acid synthesis
- Product Name:3,5-O-[(S)-phenylMethylene]-, γ-lactone D-Xylonic acid
- CAS Number:131614-83-0
- Molecular formula:C12H12O5
- Molecular Weight:236.2207
Yield:131614-83-0 33%
Reaction Conditions:
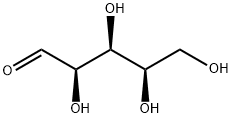
Stage #1: D-xylosewith bromine;potassium carbonate in water at 0 - 20; for 2 h;
Stage #2: benzaldehydewith sulfuric acid in tetrahydrofuran at 0 - 20;
Steps:
4.2 Synthetic procedures 4.2.1 (2S,4aR,7R,7aR)-7-Hydroxy-2-phenyltetrahydro-6H-furo[3,2-d][1,3]dioxin-6-one (13)
K2CO3 (12g, 84mmol, 1.2eq) was added in portions to a solution of D-xylose (11g, 70mmol) in water (50mL). After cooling the solution to 0°C, bromine (3.9mL, 12g, 77mmol, 1.1eq) was added slowly to the vigorously stirring solution. The reaction mixture was stirred for 30min at 0°C and for additional 90min at ambient temperature. Then, formic acid (2mL) was added, leading to the decolourisation of the solution after about 15min, whereupon the solvent was evaporated in vacuo. The residue was dissolved in dry THF (100mL), the mixture was cooled to 0°C, and benzaldehyde (21mL, 22g, 210mmol, 3eq) and conc. H2SO4 (2mL) were added. After stirring the mixture at ambient temperature overnight, it was carefully neutralized using a saturated aqueous solution of NaHCO3 and extracted with ethyl acetate (2×). The combined organic layers were dried (Na2SO4), filtered, and the solvent was removed in vacuo. The residue was purified by flash column chromatography (=8cm, h=20cm, V=65mL, petroleum ether/ethyl acetate=1/1, Rf=0.35) to give 13 as colorless solid (5.4g, 23mmol, 33% yield). m.p.: 122°C; [α]20D [α]D20 =+60.5 (3.8, methanol); 1H NMR (400MHz, DMSO-d6): δ [ppm]=4.00 (d, J=5.0Hz, 1H, 7-H), 4.26 (dd, J=13.7/2.0Hz, 1H, 4-H), 4.40 (d, J=13.7Hz, 1H, 4-H), 4.59 (d, J=2.3Hz, 1H, 7a-H), 4.65-4.69 (m, 1H, 4a-H), 5.69 (s, 1H, 2-H), 6.69 (d, J=5.0Hz, 1H, OH), 7.34-7.42 (m, 5H, Hphenyl); 13C NMR (101MHz, DMSO-d6): δ [ppm]=65.7 (1C, C-4), 72.7 (1C, C-7), 73.4 (1C, C-4a), 76.7 (1C, C-7a), 97.9 (1C, C-2), 126.0 (2C, C-2′phenyl, C-6′phenyl), 128.1 (2C, C-3′phenyl, C-5′phenyl), 129.0 (1C, C-4′phenyl), 137.6 (1C, C-1′phenyl), 175.3 (1C, C-6); IR (neat): ν ν [cm-1]=3331, 2924, 2853, 1758, 1392, 1192, 1128, 1075, 1057, 987, 929, 906, 835, 760, 748, 699, 642, 522, 462; HRMS (m/z): [M+H]+ calcd for C12H13O5: 237.0757, found: 237.0752; HPLC (method 1): tR=16.9min, purity 99.4%.
References:
Dreger, Alexander;Hoff, Katharina;Agoglitta, Oriana;Hotop, Sven-Kevin;Br?nstrup, Mark;Heisig, Peter;Kirchmair, Johannes;Holl, Ralph [Bioorganic Chemistry,2021,vol. 107,art. no. 104603]

100-52-7
944 suppliers
$20.37/250g
15384-37-9
43 suppliers
$110.00/250mg
![3,5-O-[(S)-phenylMethylene]-, γ-lactone D-Xylonic acid](/CAS/20180808/GIF/131614-83-0.gif)
131614-83-0
7 suppliers
inquiry
1125-88-8
349 suppliers
$6.00/25g

15384-37-9
43 suppliers
$110.00/250mg
![3,5-O-[(S)-phenylMethylene]-, γ-lactone D-Xylonic acid](/CAS/20180808/GIF/131614-83-0.gif)
131614-83-0
7 suppliers
inquiry

100-52-7
944 suppliers
$20.37/250g
![3,5-O-[(S)-phenylMethylene]-, γ-lactone D-Xylonic acid](/CAS/20180808/GIF/131614-83-0.gif)
131614-83-0
7 suppliers
inquiry
15384-34-6
58 suppliers
$85.00/1g
![3,5-O-[(S)-phenylMethylene]-, γ-lactone D-Xylonic acid](/CAS/20180808/GIF/131614-83-0.gif)
131614-83-0
7 suppliers
inquiry