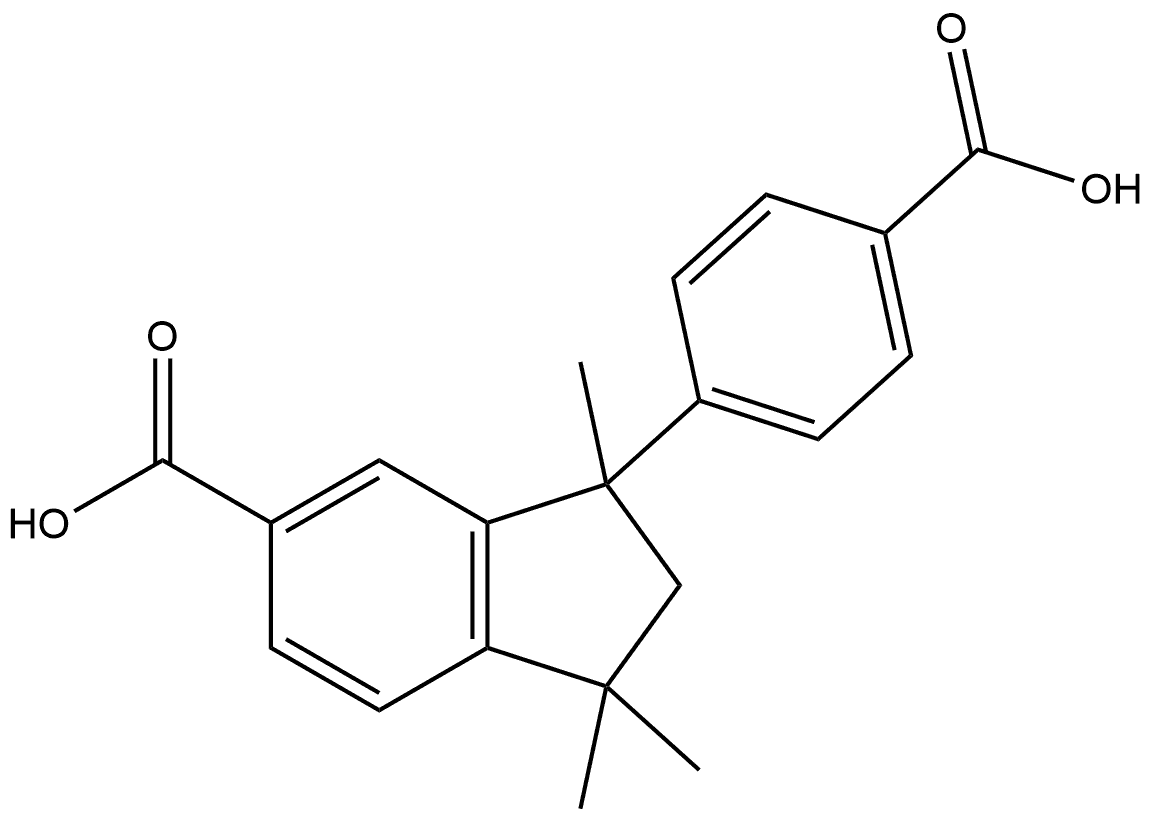
1H-Indene-5-carboxylic acid, 3-(4-carboxyphenyl)-2,3-dihydro-1,1,3-trimethyl-, (+)- synthesis
- Product Name:1H-Indene-5-carboxylic acid, 3-(4-carboxyphenyl)-2,3-dihydro-1,1,3-trimethyl-, (+)-
- CAS Number:132036-33-0
- Molecular formula:C20H20O4
- Molecular Weight:324.37
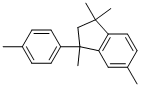
1153-36-2
4 suppliers
inquiry

132036-33-0
0 suppliers
inquiry
Yield:132036-33-0 7.2 g
Reaction Conditions:
Stage #1: 1-(4-methylphenyl)-1,3,3,6-tetramethylindanewith nitric acid for 43 h;Reflux;
Stage #2: with potassium permanganate;sodium hydroxide for 1 h;Reflux;Reagent/catalyst;Temperature;
Steps:
3 Oxidation of Dimer to PIDA
In a clean RB flask, Dimer (6.2 g, 0.023 mol) wastaken and added conc. HNO3 (50%) 30 ml, and the resulting mixture was heated to reflux for about 43 h, then cooled to room temperature and the solid thus formed was filtered, washed with water and dried completely. To the crude product, 1 N NaOH solution (120 ml) was added followed by KMnO4 (8 g) and the resultant mixture was then heated to reflux for about 1 hr. Then cooled to room temperature, acidified with conc. HC1 (pH<2). The solid thus obtained was filtered, washed with water and dried to get PIDA (7.2 g).
References:
US2015/336869,2015,A1 Location in patent:Paragraph 0302; 0303; 0310; 0311
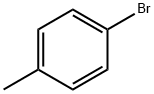
106-38-7
399 suppliers
$14.00/5g

132036-33-0
0 suppliers
inquiry
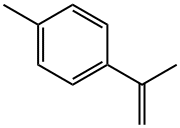
1195-32-0
95 suppliers
$53.20/5g

132036-33-0
0 suppliers
inquiry

99-87-6
237 suppliers
$14.00/25mL

132036-33-0
0 suppliers
inquiry
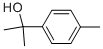
1197-01-9
75 suppliers
$54.60/sample-k

132036-33-0
0 suppliers
inquiry