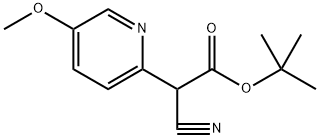
2-Pyridineacetic acid, α-cyano-5-methoxy-, 1,1-dimethylethyl ester synthesis
- Product Name:2-Pyridineacetic acid, α-cyano-5-methoxy-, 1,1-dimethylethyl ester
- CAS Number:1334784-81-4
- Molecular formula:C13H16N2O3
- Molecular Weight:248.28
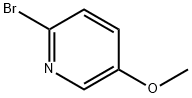
105170-27-2
191 suppliers
$8.00/1g
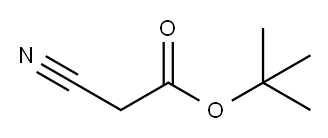
1116-98-9
238 suppliers
$8.00/5g

1334784-81-4
4 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: cyanoacetic acid tert-butyl esterwith potassium tert-butylate in tetrahydrofuran;1,4-dioxane; for 0.0833333 h;Inert atmosphere;
Stage #2: 2-bromo-5-methoxypyridine;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in tetrahydrofuran;1,4-dioxane at 70;
Steps:
A solution of tert-butyl cyanoacetate (3.75 mL, 26.2 mmol) in 20 mL of anhydrous dioxane was purged with nitrogen. To the solution was added 21.5 mL of potassium tert-butoxide (21.5 mmol, 1M in THF). After stirring for 5 minutes, 2-bromo-5-methoxypyridine (2 g, 10.7 mmol) dissolved in 4 mL of dioxane was added followed by 1,1'-bis(diphenylphosphino)ferrocene palladium dichloride-CH2Cl2 (1:1 complex) (239 mg, 0.29 mmol). The mixture was heated at 70° C. overnight after which a second aliquot of catalyst (120 mg, 0.15 mmol) was added. After heating for an additional 3 hours the mixture was cooled to room temperature and 2N acetic acid (80 mL) was added. The mixture was filtered, washing with water (2×) and the obtained crude product was dried in a current of air (1.22 g). The filtrate was extracted twice with ethyl acetate. The combined extracts were washed with brine, dried over anhydrous sodium sulfate and concentrated to a black oil which was combined with the above solids to yield 3.46 g of crude material. This material was used directly in the next step.
References:
US2011/230461,2011,A1 Location in patent:Page/Page column 28