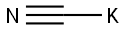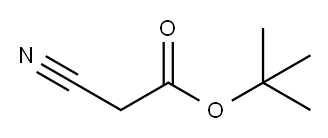
tert-Butyl cyanoacetate synthesis
- Product Name:tert-Butyl cyanoacetate
- CAS Number:1116-98-9
- Molecular formula:C7H11NO2
- Molecular Weight:141.17
1H NMR (400 MHz, CDCl 3 ) δ 3.37 (s, 2H), 1.50 (s, 9H).

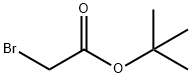
372-09-8
350 suppliers
$20.00/25g
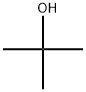
75-65-0
740 suppliers
$10.00/10ml

1116-98-9
243 suppliers
$8.00/5g
Yield:1116-98-9 86%
Reaction Conditions:
with dmap;dicyclohexyl-carbodiimide in dichloromethane at 0 - 20; for 5 h;Inert atmosphere;Reagent/catalyst;Temperature;
Steps:
7
Under argon, cyanoacetic acid (7.16 mmol) and t-butanol (6.51 mmol) are dissolved in anhydrous dichloromethane (30 mL) and cooled to 0° C. DCC (7.16 mmol) and DMAP (cat.) are added to the solution. The medium is stirred for 4 hours at 0° C. and 1 h at room temperature. The reaction mixture is filtered, then concentrated under reduced pressure. The thereby obtained is purified by column chromatography (SiO2, 20% EtOAccyclohexane) in order to obtain the t-butyl ester as a colorless oil (86%). [0501] 1H NMR (400 MHz, CDCl3): δ 4.23 (q, J=6.5 Hz, 2H, H-3), 3.44 (s, 2H, H-2), 1.28 (t, J=6.5 Hz, 3H, H-4).
References:
Ducki, Sylvie;Bennis, Khalil;Eschalier, Alain;Busserolles, Jérôme;Lesage, Florian;Rodriguez, Nuno;Vivier, Delphine US2015/38466, 2015, A1 Location in patent:Paragraph 0500-0501

372-09-8
350 suppliers
$20.00/25g
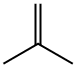
115-11-7
229 suppliers
$45.00/25 g

1116-98-9
243 suppliers
$8.00/5g