
N-((5-(4-chloroquinazolin-6-yl)furan-2-yl)Methyl)-2-(Methylsulfonyl)ethanaMine synthesis
- Product Name:N-((5-(4-chloroquinazolin-6-yl)furan-2-yl)Methyl)-2-(Methylsulfonyl)ethanaMine
- CAS Number:1334953-75-1
- Molecular formula:C16H16ClN3O3S
- Molecular Weight:365.83
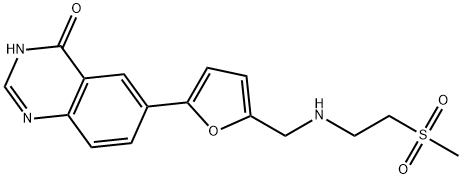
1334953-74-0
10 suppliers
inquiry

1334953-75-1
13 suppliers
inquiry
Yield:1334953-75-1 95%
Reaction Conditions:
Stage #1: 6-[5-({[2-(methylsulphonyl)ethyl]amino}methyl)furan-2-yl]quinazolin-4-olwith trichlorophosphate in toluene at 20; for 0.166667 h;Inert atmosphere;
Stage #2: with triethylamine in toluene at 30 - 75; for 3 h;
Steps:
5
Example 5 - Preparation of the intermediate N-{[5-(4-chloroquinazoline-6-yl)furan-2-yl]methyl}-2-(methylsulfonyl)ethanamine of formula (VII) - exemplifying the inventionSynthesis scheme [Show Image] In a 4-neck glass flask provided with a mechanical stirrer, condenser and thermometer, the entirety having been previously dried, 63.8 g of 6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)furan-2-yl] quinazoline-4-ol of formula (VI)(0.184 moles), 33.7 g (20.1 mL) of phosphoryl oxychloride (POCl3; 0.220 moles) and 150 mL of toluene were introduced, under nitrogen. Stirring is carried out at ambient temperature for 10 minutes then 22.3 g (30.7 mL) of triethylamine (0.220 moles) are dosed, maintaining the T below 30°C. After introduction heating is carried out at 75°C for 3 hours. The reaction is controlled by means of TLC using the Hexane/Ethyl acetate mixture (4:6) as eluent. Cooling is carried out at 0°C and stirring is carried out for an hour at such temperature. The suspension is filtered washing the solid with 100 mL of toluene. The product is dried at 50°C for 7-8 hours under vacuum. There are obtained 63.8 g of product with a molar yield equivalent to 95%.
References:
EP2468745,2012,A1 Location in patent:Page/Page column 12

1334953-74-0
10 suppliers
inquiry

1334953-75-1
13 suppliers
inquiry
16064-08-7
225 suppliers
$10.00/1g

1334953-75-1
13 suppliers
inquiry
![2-Furanmethanamine, N-[2-(methylsulfonyl)ethyl]-5-[4-[(tetrahydro-2H-pyran-2-yl)oxy]-6-quinazolinyl]-](/CAS/20210111/GIF/1383531-71-2.gif)
1383531-71-2
1 suppliers
inquiry

1334953-75-1
13 suppliers
inquiry
![2-Furancarboxaldehyde, 5-[4-[(tetrahydro-2H-pyran-2-yl)oxy]-6-quinazolinyl]-](/CAS/20210111/GIF/1383531-70-1.gif)
1383531-70-1
1 suppliers
inquiry

1334953-75-1
13 suppliers
inquiry