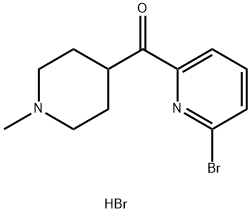
Methanone, (6-bromo-2-pyridinyl)(1-methyl-4-piperidinyl)-, hydrobromide (1:1) synthesis
- Product Name:Methanone, (6-bromo-2-pyridinyl)(1-methyl-4-piperidinyl)-, hydrobromide (1:1)
- CAS Number:1338000-32-0
- Molecular formula:C12H16Br2N2O
- Molecular Weight:364.08
Yield: 80%
Reaction Conditions:
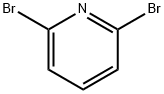
Stage #1:2,6-Dibromopyridine with n-butyllithium in hexane;tert-butyl methyl ether at -58; for 4 h;
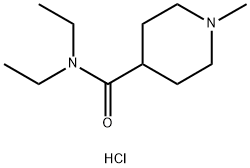
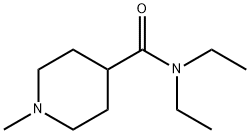
Stage #2:N,N-diethyl-1-methylpiperidine-4-carboxamide hydrochloride with sodium hydroxide in hexane;tert-butyl methyl ether;water at 0; for 2.25 h;
Stage #3: with hydrogen bromide in water;butan-1-ol for 1.5 h;
Steps:
C Scheme 1, step C
A suspension of N,N-di ethyl- 1-methyl-piperi dine-4- carboxamide hydrate hydrochloride (21.5 g, 85.1 mmol) in MTBE (109 mL) is treated with a 20 weight % aqueous solution of NaOH (34.0 g, 170 mmol). A water rinse (1.94 mL) is used to complete the addition. The mixture is stirred at RT for 30 min, the phases are allowed to settle, and phases are separated. The aqueous phase is extracted with MTBE (43.7 mL) and the organic phases combined. The organic phase is dried by distillation at atmospheric pressure until the in process control for water content by Karl- Fischer analysis is <0.10 weight %. If the target analysis is not met, the reaction is charged with MTBE (43.7 mL) and the distillation is repeated. Typically three (0122) distillations are required to reach the target analysis for water. In a separate reactor is charged a mixture of 2,6-dibromopyridine (30.2 g, 128 mmol) and MTBE (105 mL) and is cooled to less than -58 °C. To the cooled suspension is charged a 2.5M solution of n- BuLi in hexanes (51.3 mL, 128 mmol) over a 2 hr period. A rinse of MTBE (4.5 mL) is used to complete the transfer. The mixture is aged while maintaining the temperature at less than -58 °C for an additional 2 hr after complete n- BuLi addition. After aging, the solution of N,N-di ethyl- l-methyl-piperidine-4-carboxamide hydrate hydrochloride in MTBE is added to the cold reaction over a 45 min period. A rinse of MTBE (13.5 mL) is used to complete the transfer. The mixture is aged for at least 30 min after complete addition of N,N-di ethyl- l-methyl-piperidine-4-carboxamide hydrate hydrochloride in MTBE. After aging, the reaction is warmed to 0 °C over 1 hr. The cold reaction mixture is added to a 2.5 M aqueous solution of HC1 (146 mL, 366 mmol) at a rate to maintain the quench temperature at NMT 30 °C. A rinse of MTBE (13.5 mL) is used to complete the transfer. The mixture is stirred for at least 30 min after the transfer is complete and the phases are allowed to settle. The phases are separated and the aqueous phase is retained. -BuOH (54.8 mL) is added to the aqueous phase and the mixture is treated with a 20 weight % aqueous solution of NaOH (59.5 g, 298 mmol). A rinse of water (2.80 mL) is used to complete the transfer. The mixture is stirred for at least 30 min and the phases are allowed to settle. The phases are separated and the organic phase is retained. The aqueous phase is extracted with -BuOH (54.8 mL). The combined organic phases are dried by distillation under vacuum to obtain an in process control for water content by Karl- Fischer analysis of <0.20 weight %. If the target analysis is not met, //-BuOH (41.1 mL) is charged and the distillation is repeated. Typically, two distillations are required to reach the in process control target analysis. The concentrated solution is clarified by filtration and a rinse with -BuOH (89.6 mL) is used to complete the transfer and rinse the filter. The clarified solution is treated with a 48 weight % aqueous solution of HBr (9.91 mL, 87.7 mmol) over a 90 min period. A rinse of -butanol (13.8 mL) is used to complete the transfer. A check of the pH shows the reaction mixture has a pH ~1. The mixture is dried by distillation at atmospheric pressure to obtain an in process control for water content by Karl-Fischer analysis of <0.30 weight %. The mixture is concentrated to 172 mL. If the target analysis is not met, //-BuOH (54.8 mL) is charged and the distillation is repeated. The mixture is cooled to 20 °C and stirred for 12 hr. The resulting solids are collected by filtration and washed twice with -BuOH (10.75 mL). The solids are dried under vacuum at 60 °C to obtain the title compound (24.8 g, 80% yield). MS (m/z): 283, 285 (79Br, 81Br, M+H).
References:
ELI LILLY AND COMPANY WO2021/7155, 2021, A1 Location in patent:Page/Page column 30-31
498-94-2
1 suppliers
$5.00/10g

1338000-32-0
22 suppliers
inquiry

71985-80-3
151 suppliers
$6.00/1g

1338000-32-0
22 suppliers
inquiry