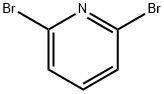
2,6-Dibromopyridine
- CAS No.
- 626-05-1
- Chemical Name:
- 2,6-Dibromopyridine
- Synonyms
- 2,4-dibroMo-6-nitropyridine;2,6-dibromo-pyridin;2,6-DIBROMOPYRIDINE;Lasmiditan Impurity 46;Pyridine, 2,6-dibromo-;2,6-Dibromopyridine,98%;2,6-Dibromopyridine >2,6-DibroMopyridine, 98% 25GR;2,6-Dibromopyridine CAS 626-05-1;2,6-Dibromopyridine ISO 9001:2015 REACH
- CBNumber:
- CB2299002
- Molecular Formula:
- C5H3Br2N
- Molecular Weight:
- 236.89
- MDL Number:
- MFCD00006223
- MOL File:
- 626-05-1.mol
- MSDS File:
- SDS
| Melting point | 117-119 °C (lit.) |
|---|---|
| Boiling point | 255°C |
| Density | 2.0383 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| Flash point | 213 °C |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | -3.65±0.10(Predicted) |
| form | Crystalline Powder |
| color | White to gray or buff |
| Water Solubility | insoluble |
| BRN | 108922 |
| InChI | InChI=1S/C5H3Br2N/c6-4-2-1-3-5(7)8-4/h1-3H |
| InChIKey | FEYDZHNIIMENOB-UHFFFAOYSA-N |
| SMILES | C1(Br)=NC(Br)=CC=C1 |
| CAS DataBase Reference | 626-05-1(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | D9SBA5R353 |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H312+H332-H315-H319-H335 | |||||||||
| Precautionary statements | P280-P301+P310+P330-P302+P352+P312-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,T,T+ | |||||||||
| Risk Statements | 36/37/38-25-20/21-52-28-52/53-36/38 | |||||||||
| Safety Statements | 26-37/39-45-36/37/39-36-36/37-28-61 | |||||||||
| RIDADR | 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | US7883000 | |||||||||
| Hazard Note | Irritant | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29333999 | |||||||||
| NFPA 704 |
|
2,6-Dibromopyridine price More Price(50)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D43115 | 2,6-Dibromopyridine 98% | 626-05-1 | 25g | $118 | 2024-03-01 | Buy |
| Sigma-Aldrich | D43115 | 2,6-Dibromopyridine 98% | 626-05-1 | 100g | $208 | 2024-03-01 | Buy |
| TCI Chemical | D1555 | 2,6-Dibromopyridine >98.0%(GC) | 626-05-1 | 25g | $34 | 2024-03-01 | Buy |
| TCI Chemical | D1555 | 2,6-Dibromopyridine >98.0%(GC) | 626-05-1 | 250g | $230 | 2024-03-01 | Buy |
| Alfa Aesar | A15397 | 2,6-Dibromopyridine, 98% | 626-05-1 | 25g | $72.6 | 2024-03-01 | Buy |
2,6-Dibromopyridine Chemical Properties,Uses,Production
Chemical Properties
White to light yellow crystalline powder
Uses
2,6-Dibromopyridine is used as a tridentate chelating ligand and in the formation of macrocycles containing the terpyridine moiety. It is also used to produce 6-bromo-2-methoxypyridine.
Application
2,6-Dibromopyridine is an important organic chemical reagent with a wide range of uses:
(1) Spectroscopic studies: There is a satisfactory correlation between the normal Raman spectra of 2,6-dibromopyridine in aqueous solution and the surface enhanced Raman (SER) spectra in silver-pure sols. In the SER spectra, the compounds are notable for the stretching of the vibrational modes of (py)CBr and (CC,CN)(py) in 2,6-dibromopyridine to give enhanced vibrational intensities at 1175 and 1369 cm-1 , respectively[1].
(2) Reaction reagent: Friedel–Crafts-type acylation of alkenes with acyl chlorides has been successfully conducted with a wide substrate scope by the combined use of AlCl3 and 2,6-dibromopyridine. Trisubstituted alkenes afford allylketones or vinylketones depending on the presence or absence of hydrogen atom(s) at the β-position to the acylation site, while monosubstituted alkenes exclusively afford vinylketones[2]. In addition, 2,6-Dibromopyridine can be brominated to form 2,4,6-tribromopyridine by reacting with a mixture of bromine at 450~ 550 °C[3]. It can also be lithiated with butyl lithium for the synthesis of L-739,010[4].
(3) A selective palladium-catalysed arylation of 2,6-dibromopyridine has been developed by employing N-heterocyclic carbene ligands. Selective mono-arylation was performed in water/acetonitrile solvent system at ambient temperature with catalyst loading of 0.1 mol%. This reaction was also found to proceed smoothly in water although at a slightly elevated temperature of 80 °C. 2,6-Disubstituted and diversely substituted 2,6-pyridines were also obtained in high yields which will be of importance to organic and medicinal chemists[5].
Purification Methods
Purify 2,6-dibromopyridine by steam distillation, then recrystallise it twice from EtOH. It does not form an HgCl2 salt. [den Hertog & Wibaut Recl Trav Chim Pays Bas 51 381 1932, Beilstein 20/5 V 435.]
References
[1] S. CHATTOPADHYAY S. K B. Surface-enhanced Raman spectroscopy of 2,5-dibromopyridine and 2,6-dibromopyridine[J]. Spectrochimica acta. Part A: Molecular spectroscopy, 1992. DOI:10.1016/0584-8539(92)80253-S.
[2] SHINYA TANAKA*. Acylation of Alkenes with the Aid of AlCl3 and 2,6-Dibromopyridine[J]. Organic Letters, 2019. DOI:10.1021/acs.orglett.9b02688.
[3] H. J. HERTOG C. R K ;W Combe. Substitution reactions in the pyridine nucleus at elevated temperatures (I). The bromination of 2,6‐dibromopyridine[J]. Recueil des Travaux Chimiques des Pays-Bas, 2010. DOI:10.1002/RECL.19580770109.
[4] D. CAI T. V D Hughes. A study of the lithiation of 2,6-dibromopyridine with butyllithium, and its application to synthesis of L-739,010[J]. Tetrahedron Letters, 1996. DOI:10.1016/0040-4039(96)00336-X.
[5] PRAJAPATI D, SCHULZKE C, KINDERMANN M K, et al. Selective palladium-catalysed arylation of 2,6-dibromopyridine using N-heterocyclic carbene ligands?[J]. RSC Advances, 2015. DOI:10.1039/C5RA10561G.
2,6-Dibromopyridine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Changzhou Jiuwu Chemical co., Ltd. | +86-0519-85121086 +8613775162768 | info@jiuwuchem.cn | China | 77 | 58 |
| Taizhou Creating Bio-pharm co., ltd. | +8613586099526 | post@creatingbiopharm.com | China | 217 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 880 | 50 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29860 | 58 |
| Anhui Dexinjia Biopharm Co., Ltd | +86-531-82371986 +86-15064117275 | sales01@jndxj.com | China | 290 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10406 | 58 |
View Lastest Price from 2,6-Dibromopyridine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-16 | 2,6-Dibromopyridine
626-05-1
|
US $19.90 / kg | 1kg | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-04-02 | 2,6-Dibromopyridine
626-05-1
|
US $15.00-50.00 / kg | 1kg | NLT98% | 5 ton per month | Anhui Dexinjia Biopharm Co., Ltd | |
 |
2025-03-21 | 2,6-Dibromopyridine
626-05-1
|
US $100.00 / kg | 1kg | 99 | 300tons | Hebei Mujin Biotechnology Co.,Ltd |
-

- 2,6-Dibromopyridine
626-05-1
- US $19.90 / kg
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- 2,6-Dibromopyridine
626-05-1
- US $15.00-50.00 / kg
- NLT98%
- Anhui Dexinjia Biopharm Co., Ltd
-

- 2,6-Dibromopyridine
626-05-1
- US $100.00 / kg
- 99
- Hebei Mujin Biotechnology Co.,Ltd
626-05-1(2,6-Dibromopyridine)Related Search:
1of4