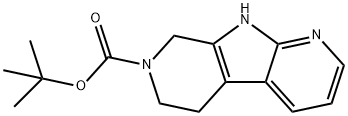
7H-Pyrrolo[2,3-b:5,4-c']dipyridine-7-carboxylic acid, 5,6,8,9-tetrahydro-, 1,1-dimethylethyl ester synthesis
- Product Name:7H-Pyrrolo[2,3-b:5,4-c']dipyridine-7-carboxylic acid, 5,6,8,9-tetrahydro-, 1,1-dimethylethyl ester
- CAS Number:1354801-07-2
- Molecular formula:C15H19N3O2
- Molecular Weight:273.33

24424-99-5
825 suppliers
$13.50/25G
![5H-Pyrrolo[2,3-b:5,4-c']dipyridine, 6,7,8,9-tetrahydro-, hydrochloride](/CAS/20180808/GIF/1354801-06-1.gif)
1354801-06-1
16 suppliers
inquiry
![7H-Pyrrolo[2,3-b:5,4-c']dipyridine-7-carboxylic acid, 5,6,8,9-tetrahydro-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1354801-07-2.gif)
1354801-07-2
3 suppliers
inquiry
Yield:1354801-07-2 72.1 g
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;water at 20; for 12.5 h;Cooling with ice;
Steps:
8 Example 8 tert-butyl 5,6,8,9-tetrahydro-7H-pyrido[4′,3′:4,5]pyrrolo[2,3-b]pyridine-7-carboxylate
The compound (95.9 g) produced in Example 7 was suspended in 1,4-dioxane (1.94 L), and a 1M aqueous sodium hydroxide solution (480 mL, 0.48 mol) was added. This solution was cooled with ice, di-tert-butyl dicarbonate (104.8 g) was added, and the mixture was stirred at room temperature for 12.5 hours. The reaction solution was placed into an aqueous saturated sodium dicarbonate solution (6 L), followed by extraction with ethyl acetate (2 L) three times. The extract solution was washed with an aqueous saturated sodium chloride solution (2 L), dried using anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting pale brown solid was treated with a silica gel column (ethyl acetate) to collect target fractions. The solvent was distilled off under reduced pressure, hexane (880 mL) was added, and this was mixed, and allowed to stand overnight at room temperature. A crystal was filtered off, washed using a mixed solution (150 mL) of hexane:ethyl acetate (10:1) and dried at room temperature under reduced pressure to obtain the title compound (72.1 g) having the following physical property values.; TLC: Rf 0.60 (chloroform:methanol:28% aqueous ammonia=90:10:1); 1H-NMR (CDCl3): δ 1.51 (s, 9H), 2.79 (t, J=6.0 Hz, 2H) 3.79 (t, J=6.0 Hz, 2H) 4.71 (s, 2H), 7.05 (dd, J=8.00, 5.00 Hz, 1H) 7.79 (m, 1H) 8.23 (m, 1H) 10.10-10.75 (m, 1H).
References:
US2013/109699,2013,A1 Location in patent:Paragraph 0936-0939
271-63-6
571 suppliers
$9.00/5g
![7H-Pyrrolo[2,3-b:5,4-c']dipyridine-7-carboxylic acid, 5,6,8,9-tetrahydro-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1354801-07-2.gif)
1354801-07-2
3 suppliers
inquiry
![1-(2,9-diazabicyclo[4.3.0]nona-2,4,7,10-tetraen-7-yl)-N,N-dimethyl-methanamine](/CAS/GIF/5654-92-2.gif)
5654-92-2
64 suppliers
$30.00/250mg
![7H-Pyrrolo[2,3-b:5,4-c']dipyridine-7-carboxylic acid, 5,6,8,9-tetrahydro-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1354801-07-2.gif)
1354801-07-2
3 suppliers
inquiry
![2-(2,9-diazabicyclo[4.3.0]nona-2,4,7,10-tetraen-7-yl)ethanamine](/CAS/20150408/GIF/4649-12-1.gif)
4649-12-1
46 suppliers
$108.00/100mg
![7H-Pyrrolo[2,3-b:5,4-c']dipyridine-7-carboxylic acid, 5,6,8,9-tetrahydro-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1354801-07-2.gif)
1354801-07-2
3 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine, 3-(2-nitroethyl)-](/CAS/20200611/GIF/183208-29-9.gif)
183208-29-9
0 suppliers
inquiry
![7H-Pyrrolo[2,3-b:5,4-c']dipyridine-7-carboxylic acid, 5,6,8,9-tetrahydro-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1354801-07-2.gif)
1354801-07-2
3 suppliers
inquiry