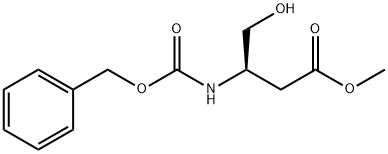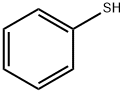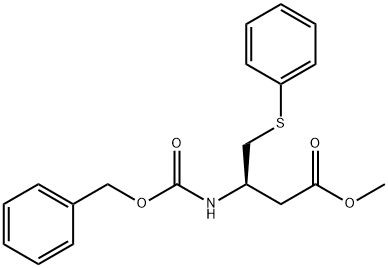
Methyl 3-(benzyloxycarbonyl)-4-(phenylthio)butanoate synthesis
- Product Name:Methyl 3-(benzyloxycarbonyl)-4-(phenylthio)butanoate
- CAS Number:1357575-01-9
- Molecular formula:C19H21NO4S
- Molecular Weight:359.44
Yield:1357575-01-9 70%
Reaction Conditions:
with tributylphosphine;1,1'-(Azodicarbonyl)dipiperidin in tetrahydrofuran; for 2.5 h;Inert atmosphere;
Steps:
5 (R)-methyl 3-(benzyloxycarbonylamino)-4-(phenylthio)butanoate
To a solution of benzenethiol (11.16 ml, 108.65 mmol), (R)-methyl 3-(benzyloxycarbonylamino)-4-hydroxybutanoate (INTERMEDIATE 4, 26.4 g, 98.77 mmol) and tributylphosphine (34.5 ml, 138.28 mmol) in anhydrous THF (350 ml) under nitrogen atmosphere was added. A solution of (E)-diazene-1,2-diylbis(piperidin-1-ylmethanone) (34.9 g, 138.22 mmol) in anhydrous THF (350 ml) was added dropwise over a 30 minute period. During the addition a white precipitate was formed and stirring was continued for 2 hours after the addition was complete. The white precipitate was filtered off and evaporation of the volatiles under reduced pressure gave a residue, which was purified by column chromatography (ISCO, eluting with 0→100% EtOAc/hexanes) to give the title product (25.0 g, yield: 70%).1H NMR (300 MHz, CHLOROFORM-d) δ ppm 2.59-2.73 (m, 1H) 2.75-2.88 (m, 1 H) 3.04-3.19 (m, 1H) 3.21-3.37 (m, 1H) 3.57-3.69 (m, 3H) 4.08-4.24 (m, 1H) 5.00-5.14 (m, 2H) 5.46 (br. s., 1H) 7.14-7.48 (m, 10H).
References:
US2012/35134,2012,A1 Location in patent:Page/Page column 33
75443-62-8
35 suppliers
$112.00/100mg

1357575-01-9
11 suppliers
inquiry
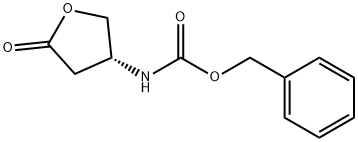
118399-28-3
136 suppliers
$6.00/100mg

1357575-01-9
11 suppliers
inquiry

123673-31-4
3 suppliers
inquiry

1357575-01-9
11 suppliers
inquiry
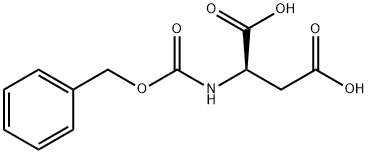
78663-07-7
226 suppliers
$5.00/1g

1357575-01-9
11 suppliers
inquiry