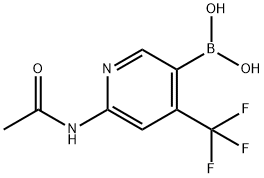
6-acetaMido-4-(trifluoroMethyl)pyridin-3-ylboronic acid synthesis
- Product Name:6-acetaMido-4-(trifluoroMethyl)pyridin-3-ylboronic acid
- CAS Number:1370351-47-5
- Molecular formula:C8H8BF3N2O3
- Molecular Weight:247.97
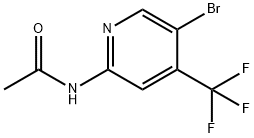
1370351-46-4
6 suppliers
inquiry

1370351-47-5
9 suppliers
inquiry
Yield:1370351-47-5 93%
Reaction Conditions:
Stage #1: N-(5-bromo-4-(trifluoromethyl)pyridin-2-yl)acetamidewith lithium amide;isopropyl alcohol in tetrahydrofuran at 2; for 1.5 h;
Stage #2: with n-butyllithium;Triisopropyl borate in tetrahydrofuran;hexane at -85 - 20;
Steps:
8.B
Process version B; To a suspension of Lithium amide (5.6 g, 233.2 mmol) in 140 ml THF was added at room temperature 2-propanol (1.3 g, 21.2 mmol). The reaction mixture was cooled to 1T=2 °C. A solution of B2 (60.0 g, 212 mmol) in 280 ml THF was added while keeping the temperature at IT 2 °C. The reaction mixture was stirred at IT=2 °C for 90 min. THF (240 ml) was added and solvent (~380 ml) was removed by distillation. THF (360 ml) was added and solvent (~380 ml) was removed by distillation. THF (200 ml) was added and the reaction mixture was cooled to IT=-85 °C. A solution (76 ml, 190 mmol) nBuLi, 2.5 M in hexane was added at -85 °C followed by addition of a solution of triisopropylborate (31.9 g, 170 mmol) in THF (17 ml). A solution (17.3 ml, 43.3 mmol) n BuLi, 2.5 in hexane was added at -85 °C followed by addition of a solution of triisopropylborate (23.9 g, 127 mmol) in THF (13 ml). The reaction mixture was allowed to warm to RT and was stirred over night at RT. Water (15.3 g) and HCI in 2-propanol, 5-6 M (36.7 g) was added at RT. Isopropylacetate (480 ml) was added and solvent was removed by distillation until a volume of ~360 ml was obtained. Isopropylacetate (480 ml) was added and solvent was removed by distillation until a volume of ~360 ml was obtained. Isopropylacetate (480 ml) was added and the mixture was cooled to IT=0 °C. Water (480 g) was added and by addition of N NaOH the pH was adjusted at IT=0 °C to pH 1. The precipitate was removed by filtration. The aquoeus layer was separated at IT=0 °C. Water was added and the aquoeus layer was separated at IT=0 °C. The combined aquoues layers were cooled to IT=0 °C and the pH was adjusted by addition of 1 N HCI to pH 3. The precipitate was collected and the dried at 30 °C/40- 45 mbar until constant weight was obtained. 48.9 g (93 %) 6-acetamido-4- (trifluoromethyl)pyridin-3-ylboronic acid B3 were obtained.
References:
WO2012/44727,2012,A2 Location in patent:Page/Page column 72
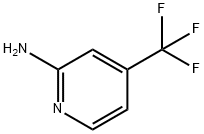
106447-97-6
348 suppliers
$11.00/1g

1370351-47-5
9 suppliers
inquiry