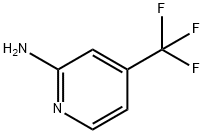
2-Amino-4-(trifluoromethyl)pyridine synthesis
- Product Name:2-Amino-4-(trifluoromethyl)pyridine
- CAS Number:106447-97-6
- Molecular formula:C6H5F3N2
- Molecular Weight:162.11
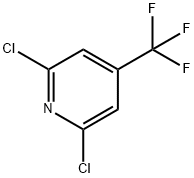
39890-98-7
183 suppliers
$13.00/1g

106447-97-6
344 suppliers
$11.00/1g
Yield:106447-97-6 71.9%
Reaction Conditions:
Stage #1:2,6-dichloro-4-(trifluoromethyl)pyridine with ammonia in tetrahydrofuran;water at 150; for 6 h;autoclave;
Stage #2: with hydrogen;5%-palladium/activated carbon in tetrahydrofuran;water at 100; under 15001.5 Torr; for 3 h;Product distribution / selectivity;
Steps:
5
Example 5 In an autoclave (200 mL), 10 g (0.046 moles) of 2,6,4-DCTF, 30.6 mL (0.44 moles) of 28 % ammonia water, and 20 mL of THF were heated to 150 °C with stirring to effect reaction. After about 6 hours, the autoclave was cooled to from 30 to 40 °C, 300 mg of 5 % Pd/C (54 % wet, 0.076 mmoles) was added thereto, hydrogen was filled up to 2.0 MPa, followed by heating to 100 °C with stirring to effect reaction. After about 3 hours, the pressure reactor was cooled to from 30 to 40 °C, followed by filtration through Celite. Water was added to the filtrate; the extraction with ethyl acetate was conducted three times; and an organic layer was washed with saturated saline and then dried over sodium sulfate. The organic layer was concentrated under reduced pressure, and n-hexane was added, followed by concentration. To a deposited crystal, n-hexane was added, followed by stirring under ice cooling for about 60 minutes, and a deposited crystal was filtered. The obtained crystal was subjected to washing with ice-cooled n-hexane three times and then dried under reduced pressure to obtain 5.4 g of 2,4-ATF as a white crystal (yield: 71.9 %, melting point: 70.1 °C). In addition, an NMR spectrum data of the obtained crystal is as follows. 1H-NMR (CDCl3): δ 4.713 (brs, 2H), 6.684 (s, 1H), 6.823 (dd, J = 5.2 and 1.2 Hz, 1H), 8.205 (d, J = 5.6 Hz, 1H)
References:
Ishihara Sangyo Kaisha, Ltd. EP2527327, 2012, A1 Location in patent:Page/Page column 7
917806-22-5
0 suppliers
inquiry

106447-97-6
344 suppliers
$11.00/1g
944401-56-3
157 suppliers
$32.00/250mg

73183-34-3
566 suppliers
$6.00/5g

944401-57-4
101 suppliers
$45.00/25mg

106447-97-6
344 suppliers
$11.00/1g

39890-98-7
183 suppliers
$13.00/1g

130171-52-7
19 suppliers
$65.00/10mg

106447-97-6
344 suppliers
$11.00/1g

81565-18-6
336 suppliers
$8.00/1g

106447-97-6
344 suppliers
$11.00/1g