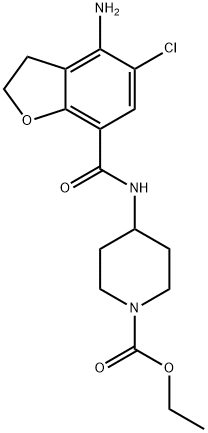
Prucalopride Impurity 10/ethyl 4-(4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxamido)piperidine-1-carboxylate synthesis
- Product Name:Prucalopride Impurity 10/ethyl 4-(4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxamido)piperidine-1-carboxylate
- CAS Number:137211-63-3
- Molecular formula:C17H22ClN3O4
- Molecular Weight:367.83
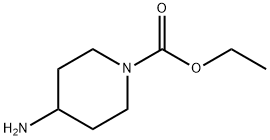
58859-46-4
218 suppliers
$13.00/5g
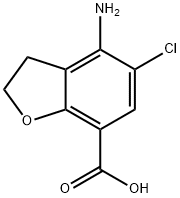
123654-26-2
186 suppliers
$12.00/100mg

137211-63-3
10 suppliers
inquiry
Yield:137211-63-3 12 g
Reaction Conditions:
Stage #1: 4-amino-5-chloro-2,3-dihydrobenzo[b]furan-7-carboxylic acidwith chloroformic acid ethyl ester;triethylamine in chloroform at 0 - 30;
Stage #2: ethyl 4-aminopiperidine-1-carboxylate in chloroform at 25 - 30;
Steps:
3 Preparation of ethyl 4-[[(4-amino-5-chloro-2,3-dihydro-7-benzofuranyl)carbonyl] amino]-l-piperidine-carboxylate
A solution of sodium hydroxide (1.9 g) in water (10 ml) was added to methyl 4-amino-5- chloro-2,3-dihydrobenzofuran-7-carboxylate (10 g) in methanol (50 ml) at 25-30°C. The resulting mixture was heated to reflux temperature and maintained for 2 hours at the same temperature. After completion of reaction, the solvent was distilled off under vacuum to produce 4-amino-5-chloro-2,3-dihydro-7-benzofuran-carboxylic acid as a solid. Chloroform (250 ml) was added to the resulting solid and then stirred for 15 minutes at 25- 30°C. The resulting mass was cooled to 0-5°C, followed by the addition of triethylamine (10 g) and ethyl chloro formate (10 g), and then stirring for 2-3 hours at the same temperature. A solution of ethyl 4-amino-l-piperidine -carboxylate (12.4 g) in chloroform (128 ml) was added to the reaction mass and the resulting mixture was maintained at 25- 30°C for overnight. After completion of reaction, the salts were filtered and the filtrate was washed subsequently with 5% NaOH solution (130 ml) and water (130 ml). The solvent was distilled off under reduced pressure to obtain a residue. Diisopropyl ether (40 ml) was added to the residue and then stirred for 1 hour at 25-30°C. The product obtained was filtered, washed with di-isopropyl ether (15 ml) and then dried the material at 55-60°C for 3 hours to produce 12 g of ethyl 4-[[(4-amino-5-chloro-2,3-dihydro-7- benzofuranyl)carbonyl]amino]-l-piperidinecarboxylate (Purity by HPLC: 99.5%; Yield: 74.2%).
References:
WO2017/137910,2017,A1 Location in patent:Page/Page column 26; 27
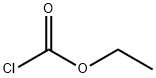
541-41-3
6 suppliers
$21.00/5g

58859-46-4
218 suppliers
$13.00/5g
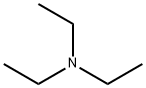
121-44-8
843 suppliers
$9.00/5g

123654-26-2
186 suppliers
$12.00/100mg

137211-63-3
10 suppliers
inquiry
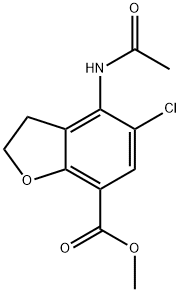
143878-29-9
103 suppliers
$15.00/100mg

137211-63-3
10 suppliers
inquiry
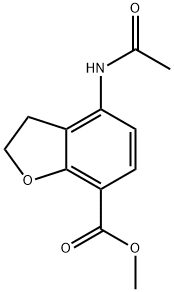
149466-67-1
30 suppliers
inquiry

137211-63-3
10 suppliers
inquiry
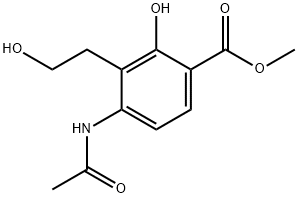
202664-85-5
14 suppliers
inquiry

137211-63-3
10 suppliers
inquiry