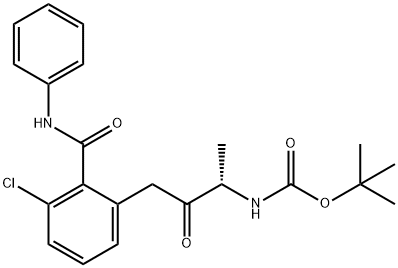
(S)-tert-butyl 4-(3-chloro-2-(phenylcarbamoyl)phenyl)-3-oxobutan-2-ylcarbamate synthesis
- Product Name:(S)-tert-butyl 4-(3-chloro-2-(phenylcarbamoyl)phenyl)-3-oxobutan-2-ylcarbamate
- CAS Number:1386861-47-7
- Molecular formula:C22H25ClN2O4
- Molecular Weight:416.9
Yield:-
Reaction Conditions:
Stage #1: anilinewith trimethylaluminum in dichloromethane; for 0.25 h;
Stage #2: (S)-tert-butyl 1-(8-chloro-1-oxo-1H-isochromen-3-yl)ethylcarbamate in dichloromethane at 20; for 3 h;
Steps:
44 Example 44
To a mixture of R2-NH2 (197 mg, 1.54 mmol) in 2 mL of DCM is added AlMe3 (0.772 ml, 1.54 mmol). The mixture is stirred for about 15 min. Then, a solution of lactone (L-4) (100 mg, 0.309 mmol) in 2 mL of DCM is added, and the reaction is stirred at RT for about 3 hours. The reaction mixture is then quenched with addition of 10 mL of Rochelle's salt and stirring for about 2 hours. The mixture is diluted with DCM, washed with brine, dried with Na2S04 and concentrated in vacuo to afford the amide (L-5) which is carried directly to the next reaction.[00793] The following isoquinolinone compounds (L-6) were made in analogous fashion to Method L using the following R2-NH2 reagents.
References:
WO2013/12915,2013,A1 Location in patent:Paragraph 00734; 00793
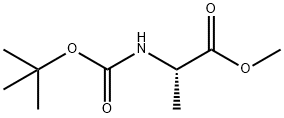
28875-17-4
142 suppliers
$6.00/5g

1386861-46-6
58 suppliers
$12.00/1g

1386861-47-7
1 suppliers
inquiry
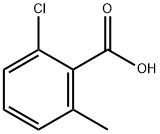
21327-86-6
252 suppliers
$6.00/250mg

1386861-47-7
1 suppliers
inquiry

15761-38-3
516 suppliers
$5.00/10g

1386861-47-7
1 suppliers
inquiry
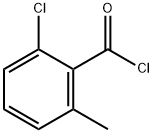
89894-44-0
46 suppliers
$21.00/100mg

1386861-47-7
1 suppliers
inquiry
![Carbamic acid, N-[(1S)-1-(8-chloro-1-oxo-1H-2-benzopyran-3-yl)ethyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1420625-96-2.gif)
