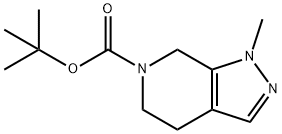
tert-Butyl 1-Methyl-4,5-dihydro-1H-pyrazolo[3,4-c]pyridine-6(7H)-carboxylate synthesis
- Product Name:tert-Butyl 1-Methyl-4,5-dihydro-1H-pyrazolo[3,4-c]pyridine-6(7H)-carboxylate
- CAS Number:1395492-96-2
- Molecular formula:C12H19N3O2
- Molecular Weight:237.3
![tert-butyl 4,5-dihydro-1H-pyrazolo[3,4-c]pyridine-6(7H)-carboxylate](/CAS2/GIF/871726-73-7.gif)
871726-73-7
106 suppliers
$45.00/50mg

74-88-4
344 suppliers
$15.00/10g
![tert-Butyl 1-Methyl-4,5-dihydro-1H-pyrazolo[3,4-c]pyridine-6(7H)-carboxylate](/CAS/20150408/GIF/1395492-96-2.gif)
1395492-96-2
15 suppliers
inquiry
Yield:1395492-96-2 97.4%
Reaction Conditions:
Stage #1: tert-butyl 1,4,5,7-tetrahydro-6H-pyrazolo[3,4-c]pyridine-6-carboxylatewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.166667 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 15; for 16 h;
Steps:
44.5 tert-butyl 1-methyl-4,5-dihydro-1H-pyrazolo[3,4-c]pyridine-6(7H)-carboxylate
The mixture of tert-butyl 4,5-dihydro- 1 H-pyrazolo [3 ,4-c]pyridine-6(7H)- carboxylate (4.0 g, 17.937 mmol) in dry DMF (100 mL) was added NaH (861 mg, 35.874 mmol) at 0 °C, after stirring at this temperature for 10 mm, Mel (3.82 g, 26.906 mmol) was added and the resulting mixture was stuffed at 15 °C for 16 h, at which time TLC showed the completion of the reaction. The reaction mixture was quenched with aq.NH4C1 (200 mL) at 0°C, diluted with water and was extracted with DCM (100 mLx3). The organic layers were dried over Na2SO4, concentrated and purified by silica gel column chromatography (PE:EA= 100:1 1:1) to give tert-butyl 1 -methyl-4,5-dihydro- 1H-pyrazolo[3,4-c]pyridine-6(7H)- carboxylate (4.1 g, yield: 97.4%) as a light yellow oil. LCMS (nilz): 182.1 [M+H]
References:
WO2015/200677,2015,A2 Location in patent:Paragraph 00744; 00745