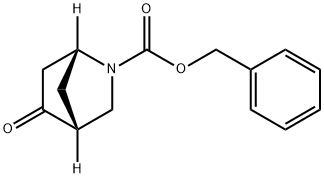
2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 5-oxo-, phenylmethyl ester, (1R,4R)- synthesis
- Product Name:2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 5-oxo-, phenylmethyl ester, (1R,4R)-
- CAS Number:1400808-09-4
- Molecular formula:C14H15NO3
- Molecular Weight:245.27
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 5-hydroxy-, phenylmethyl ester, (1R,4R,5S)-](/CAS/20200611/GIF/1400808-08-3.gif)
1400808-08-3
0 suppliers
inquiry
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 5-oxo-, phenylmethyl ester, (1R,4R)-](/CAS/20200611/GIF/1400808-09-4.gif)
1400808-09-4
6 suppliers
inquiry
Yield:1400808-09-4 75%
Reaction Conditions:
with 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione in dimethyl sulfoxide;toluene at 23 - 60; for 3.5 h;Inert atmosphere;
Steps:
B.B14
(1R,4R)-Benzyl 5-oxo-2-azabicyclo[2.2.1]heptanes-2-carboxylate (B14) 2-Iodoxybenzoic acid (13.7 g, 22.0 mmol, 45 wt. % (SIBX)) was added to a solution of (1R,4R,5S)-benzyl 5-hydroxy-2-azabicyclo[2.2.1]heptanes-2-carboxylate (4.02 g, 16.3 mmol) dissolved in dimethylsulfoxide (20.0 mL) and toluene (40.0 mL) under nitrogen atmosphere at 23° C. The mixture was stirred for 3 h 30 min at 60° C. and then cooled to 23° C. The resulting heterogeneous mixture was treated with saturated sodium carbonate (aq.) (250 mL) and filtered under reduced pressure to remove a white solid. The filtrate was extracted with ethyl acetate (250 mL*3) and the organic extracts were washed with brine, dried over magnesium sulfate and concentrated in vacuo. The crude material as colorless oil was purified by column chromatography (SiO2, EtOAc:n-Hex 1:2 (v/v)) to provide the title compound B14 (2.99 g, 12.2 mmol, 75%) as a colorless oil.
References:
US2012/238751,2012,A1 Location in patent:Page/Page column 38
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
225 suppliers
$10.00/1g
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 5-oxo-, phenylmethyl ester, (1R,4R)-](/CAS/20200611/GIF/1400808-09-4.gif)
1400808-09-4
6 suppliers
inquiry