
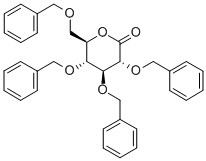
2,3,4,6-Tetra-O-benzyl-1-C-dichloromethyl-D-glucopyranose synthesis
- Product Name:2,3,4,6-Tetra-O-benzyl-1-C-dichloromethyl-D-glucopyranose
- CAS Number:140658-50-0
- Molecular formula:C35H36Cl2O6
- Molecular Weight:623.56
13096-62-3
168 suppliers
$25.00/1g

75-09-2
1200 suppliers
$10.00/25ML

140658-50-0
28 suppliers
inquiry
Yield:140658-50-0 99.6%
Reaction Conditions:
with n-butyllithium;diisopropylamine in hexane at -70 - 70; for 1 h;Temperature;Time;
Steps:
1.6 Step 6: (2R,3R,4S,5R,6R)-3,4,5-tris-benzyl-6-benzyloxymethyl-2-dichloromethyl-tetrahydro-2H-pyran-2- Synthesis of alcohol
Add 850 μL of diisopropylamine to dry 6 mL of tetrahydrofuran, cool to _5°C~-1°C in an ice salt bath, and stir underDrop 2.4 mL of n-hexane solution of butyl lithium, after dripping, react for 1-2h to obtain LDA solution.Add 1.6g of 2,3,4,6-tetra-O-benzyl-glucolactone prepared in step 5 to 15mL of dichloromethane, cool to -70°C, add LDA solution dropwise with stirring, and after the drop is completed, React at 70°C for 1h. Add 20mL 1mol/L hydrochloric acid to terminate the reaction, dilute with 200mL dichloromethane, and wash with 60mL 1mol/L hydrochloric acid, 60mL saturated sodium bicarbonate solution, 40mL saturated brine, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a pale yellow oil. The liquid was purified by silica gel column chromatography (gradient eluent: V (petroleum ether): V (ethyl acetate) = 10:1 to 6:1) to obtain 1.84 g of a pale yellow oily liquid product with a yield of 99.6%.
References:
CN113214094,2021,A Location in patent:Paragraph 0060; 0061; 0078-0081
13456-36-5
0 suppliers
inquiry

140658-50-0
28 suppliers
inquiry
604-69-3
371 suppliers
$10.00/25g

140658-50-0
28 suppliers
inquiry

131531-76-5
1 suppliers
inquiry

140658-50-0
28 suppliers
inquiry