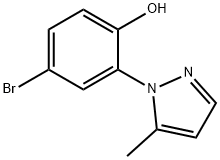
4-hydroxy-3-(5-methyl-1H-pyrazol-1- yl)benzaldehyde synthesis
- Product Name:4-hydroxy-3-(5-methyl-1H-pyrazol-1- yl)benzaldehyde
- CAS Number:1416161-80-2
- Molecular formula:C11H10N2O2
- Molecular Weight:202.21
Yield:1416161-80-2 86%
Reaction Conditions:
Stage #1: 4-bromo-2-(5-methyl-1H-pyrazol-1-yl)phenolwith methyllithium in tetrahydrofuran;1,2-dimethoxyethane at -55; for 1.75 h;Cooling with acetone-dry ice;
Stage #2: with sec.-butyllithium in tetrahydrofuran;1,2-dimethoxyethane;cyclohexane at -55; for 1.25 h;
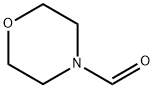
Stage #3: 4-morpholinecarboxaldehyde
Steps:
2.3
Step 3. Synthesis of 4-hydroxy-3-(5-methyl-1 /- -pyrazol-1 -yl)benzaldehyde (P2). 4-Bromo-2-(5-methyl-1 H-pyrazol-1 -yl)phenol (C15) (1 17.7 g, 464.9 mmol) was dissolved in tetrahydrofuran (1.18 L) and cooled in a dry ice/acetone bath. The resulting slurry was treated drop-wise with methyllithium (3.0 M in 1 ,2-dimethoxyethane, 232.5 ml_, 697.4 mmol) over 35 minutes, while maintaining the reaction temperature below minus 55 °C. After 1 .75 hours, sec-butyllithium (1 .4 M in cyclohexane, 498.2 ml_, 697.4 mmol) was added drop-wise over an hour, while the reaction temperature was maintained below minus 55 °C. The reaction was stirred for 15 minutes, and then morpholine-4- carbaldehyde (66.1 ml_, 658 mmol), in an equal volume of tetrahydrofuran, was added drop-wise over 20 minutes, while the reaction temperature was held below minus 55 °C. After 5 minutes, the reaction was allowed to slowly warm to minus 8 °C, at which time chilled aqueous hydrochloric acid (2 N, 465.0 ml_, 929.9 mmol) was rapidly added; the temperature was maintained below 30 °C during this addition. The organic phase was extracted with water (3 L), and the combined aqueous layers were treated drop-wise with aqueous hydrochloric acid (6 N, 77.49 ml_, 465.0 mmol) over 6 minutes. To the resulting slurry was added additional aqueous hydrochloric acid (6 N, 52.7 ml_, 316.2 mmol) over 7 minutes, and the resulting mixture was allowed to granulate under nitrogen for 18 hours. Filtration provided a solid, which was washed with water (940 ml.) and dried at 40 °C under vacuum to provide the title product as a light orange granular solid. Yield: 80.96 g, 400.4 mmol, 86%. LCMS m/z 203.1 (M+1 ). 1H NMR (400 MHz, CDCI3) δ 9.90 (s, 1 H), 7.86 (d, J=1 .8 Hz, 1 H), 7.76 (dd, J=8.4, 2.0 Hz, 1 H), 7.68 (br d, J=2.0 Hz, 1 H), 7.24 (d, J=8.3 Hz, 1 H), 6.31 -6.33 (m, 1 H), 2.54 (s, 3H).
References:
WO2012/172449,2012,A1 Location in patent:Page/Page column 33
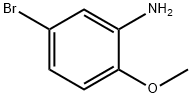
6358-77-6
167 suppliers
$6.00/250mg

1416161-80-2
5 suppliers
inquiry
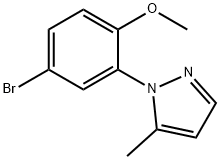
1395896-56-6
5 suppliers
inquiry

1416161-80-2
5 suppliers
inquiry