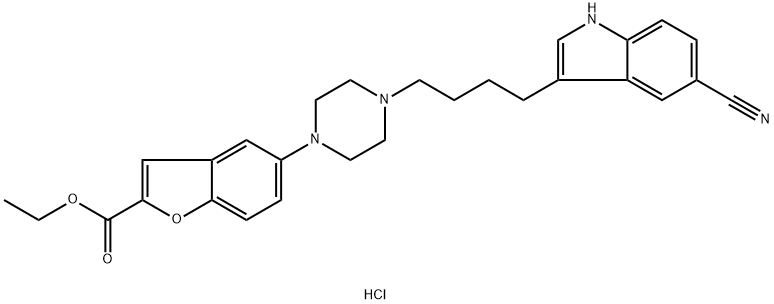
5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-2-benzofurancarboxylic acid ethyl ester hydrochlorid synthesis
- Product Name:5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-2-benzofurancarboxylic acid ethyl ester hydrochlorid
- CAS Number:1422956-25-9
- Molecular formula:C28H31ClN4O3
- Molecular Weight:507.03
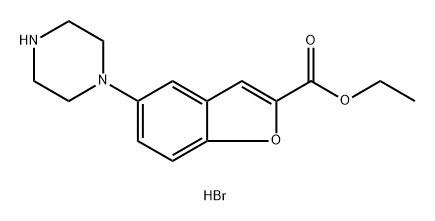
1469427-45-9
0 suppliers
inquiry
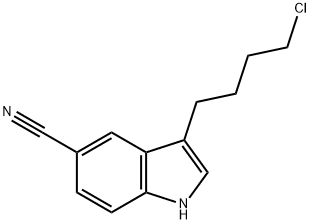
143612-79-7
327 suppliers
$23.00/1g
![5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-2-benzofurancarboxylic acid ethyl ester hydrochlorid](/CAS/20180906/GIF/1422956-25-9.gif)
1422956-25-9
5 suppliers
inquiry
Yield:1422956-25-9 83%
Reaction Conditions:
Stage #1: ethyl 5-(1-piperazinyl)benzofuran-2-carboxylate hydrobromide;3-(4-chlorobutyl)-5-cyanoindolewith tetrabutylammomium bromide;triethylamine at 85 - 90; for 10 h;
Stage #2: with hydrogenchloride in isopropyl alcohol at 50 - 55; pH=2;Reflux;
Steps:
6 Example-6: preparation of ethyl 5-(4-[4-(5-cyano-lH-indol-3- yl) butyl] piperazin-l-yl) benzofuran -2-carboxylate hydrochloride
Example-6: preparation of ethyl 5-(4-[4-(5-cyano-lH-indol-3- yl) butyl] piperazin-l-yl) benzofuran -2-carboxylate hydrochloride A mixture of ethyl 5-(l-piperazinyl)-benzofuran-2-carboxylate hydrobromide (lOOg), 3-(4- chlorobutyl) - lH-indole-5-carbonitrile(63.7g), triethyl amine (400ml) and TBAB(88.3g) were heated at 85-90°C (reflux) under stirring for 10 hrs. After completion of the reaction Acetone (400ml) was charged to the residue and reflux the reaction mass at 55-60°C under stirring for 30-45 minutes. The reaction mass was cooled to 45-50°C and activated carbon (5.0g) was charged to reaction mass and again refluxed for 15-30 minutes. The reaction mass was cooled to 15-20°C and filtered through hyflo and washed with acetone. IPA.HC1 (~ 100ml) was added to the solution at 50-55°C in more than 30 minutes to adjust the pH 2.0 under stirring and maintained for 45-60 minutes at reflux. The reaction mix was cooled to 20-30°C under stirring for 2-3 hours and then maintained at 0-5°C for 45-60 minutes under stirring. The solid was filtered and washed with Acetone. Dried at 55-60°C for 4-6 hours. Yield: 83%
References:
WO2013/153492,2013,A2 Location in patent:Page/Page column 25
69604-00-8
223 suppliers
$14.85/1gm:
![5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-2-benzofurancarboxylic acid ethyl ester hydrochlorid](/CAS/20180906/GIF/1422956-25-9.gif)
1422956-25-9
5 suppliers
inquiry

97-51-8
414 suppliers
$6.00/5g
![5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-2-benzofurancarboxylic acid ethyl ester hydrochlorid](/CAS/20180906/GIF/1422956-25-9.gif)
1422956-25-9
5 suppliers
inquiry
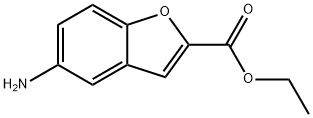
174775-48-5
248 suppliers
$8.00/250mg
![5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-2-benzofurancarboxylic acid ethyl ester hydrochlorid](/CAS/20180906/GIF/1422956-25-9.gif)
1422956-25-9
5 suppliers
inquiry