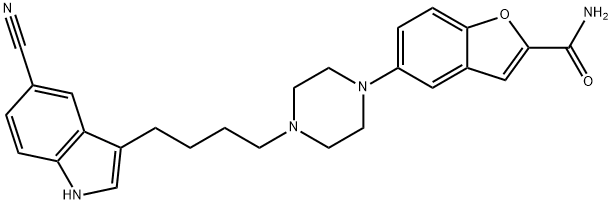
VILAZODONE synthesis
- Product Name:VILAZODONE
- CAS Number:163521-12-8
- Molecular formula:C26H27N5O2
- Molecular Weight:441.52
143612-79-7
332 suppliers
$23.00/1g

183288-46-2
219 suppliers
$15.00/250mg

163521-12-8
247 suppliers
inquiry
Yield:163521-12-8 95.7%
Reaction Conditions:
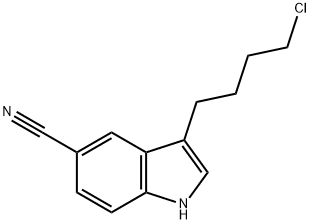
Stage #1:3-(4-chlorobutyl)-5-cyanoindole with sodium iodide in N,N-dimethyl-formamide at 25 - 30; for 0.25 h;
Stage #2:5-(1-piperazinyl)benzofuran-2-carboxamide with tetrabutylammomium bromide;sodium hydrogencarbonate in N,N-dimethyl-formamide at 110 - 115; for 1.25 h;Reagent/catalyst;Temperature;Time;Solvent;
Steps:
11.11e Preparation of Form I of vilazodone free base
To a solution of 3-(4-chlorobutyl)-1 H-indole-5-carbonitrile (2.5 g) in DMF (10 mL), sodium iodide (0.8 g) was added and the reaction mass was stirred at about 25-30 °C for 15 minutes. Sodium bicarbonate (3.61 g), 5-(piperazin-1 -yl)benzofuran-2- carboxamide (3 g) and tetrabutylammonium bromide (0.51 g) was added to the reaction mass and heated to 110-115 °C for 1 hour and 15 minutes. The reaction mass was then allowed to cool to 60 °C and water (37.5 mL) was added to the reaction mass slowly. The resulting precipitate was stirred for 60 minutes at about 30 °C. The precipitate was filtered, washed with water (10 mL) and dried for 5-6 hours at 60 °C under vacuum to afford the desired compound.Yield: 4.5 g (95.7 %). Purity (by HPLC): 97.39 %
References:
DR.REDDYS LABORATORIES LIMITED;IQBAL, Javed;ORUGANTI, Srinivas;RAPOLU, Rajesh Kumar;PEDDY, Vishweshwar;BOGE, Rajesham;PATHIVADA, Deepika;VELAGA, Dharma Jagannadha Rao;YARRAGUNTLA, Sesha Reddy;BADDAM, Sudhakar Reddy;NAREDLA, Anitha;DONIPARTHI, Kiran Kumar;NADGOUD, Ramesh Kumar;PAGADALA, Narasimha Rao;UNNIARAN, Syam Kumar;RAMAKRISHNAN, Srividya WO2013/168126, 2013, A1 Location in patent:Page/Page column 75-78
![5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-2-benzofurancarboxylic acid ethyl ester hydrochlorid](/CAS/20180906/GIF/1422956-25-9.gif)
1422956-25-9
4 suppliers
inquiry

163521-12-8
247 suppliers
inquiry
![2-Benzofurancarboxylic acid, 5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-, methyl ester](/CAS/20210305/GIF/1236722-93-2.gif)
1236722-93-2
0 suppliers
inquiry

163521-12-8
247 suppliers
inquiry