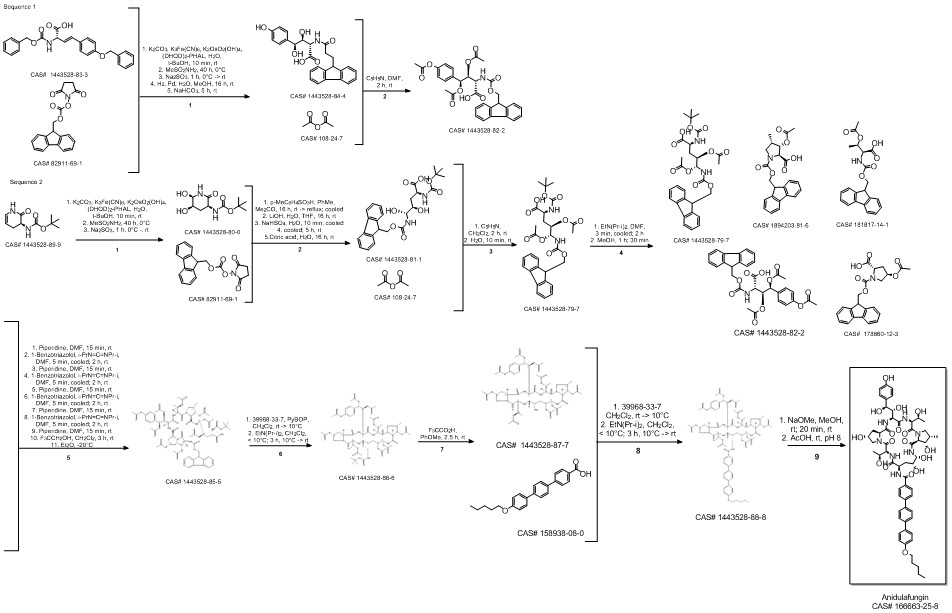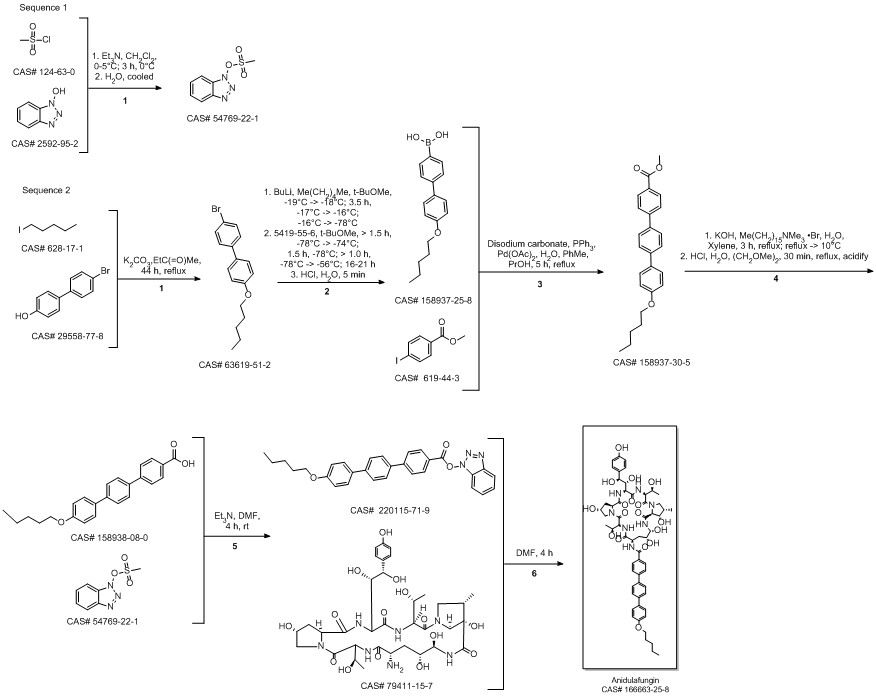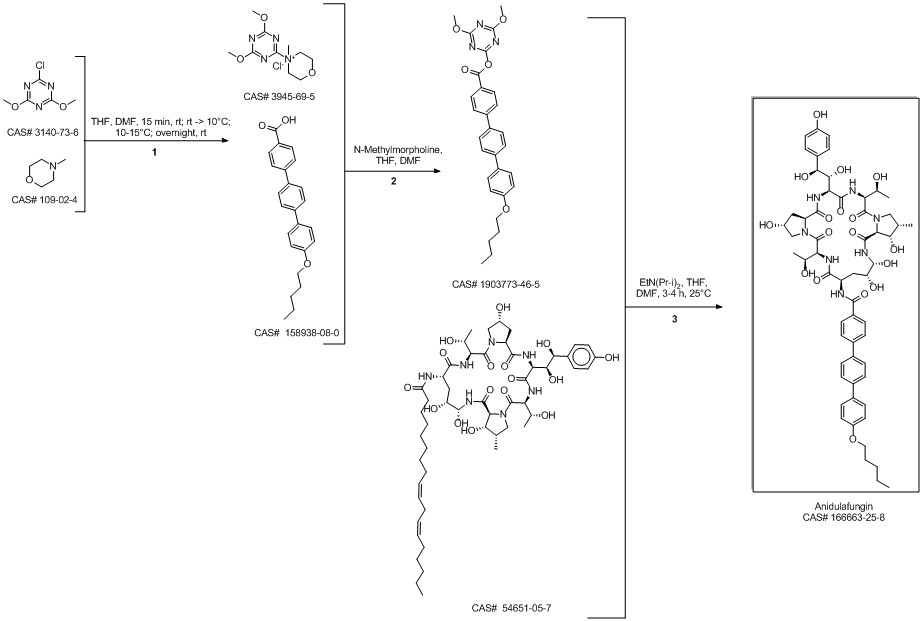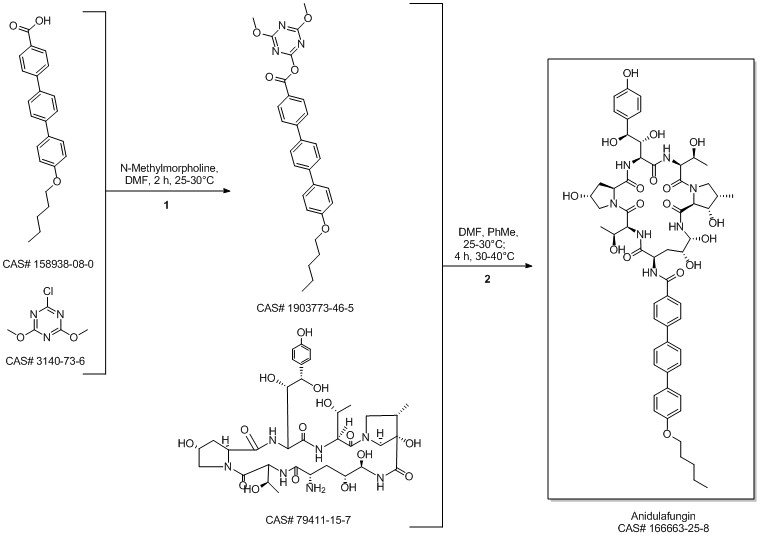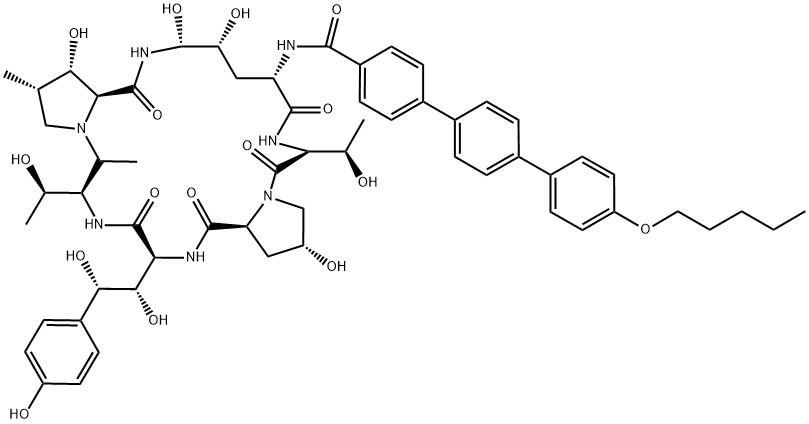
Anidulafungin synthesis
- Product Name:Anidulafungin
- CAS Number:166663-25-8
- Molecular formula:C58H73N7O17
- Molecular Weight:1140.24
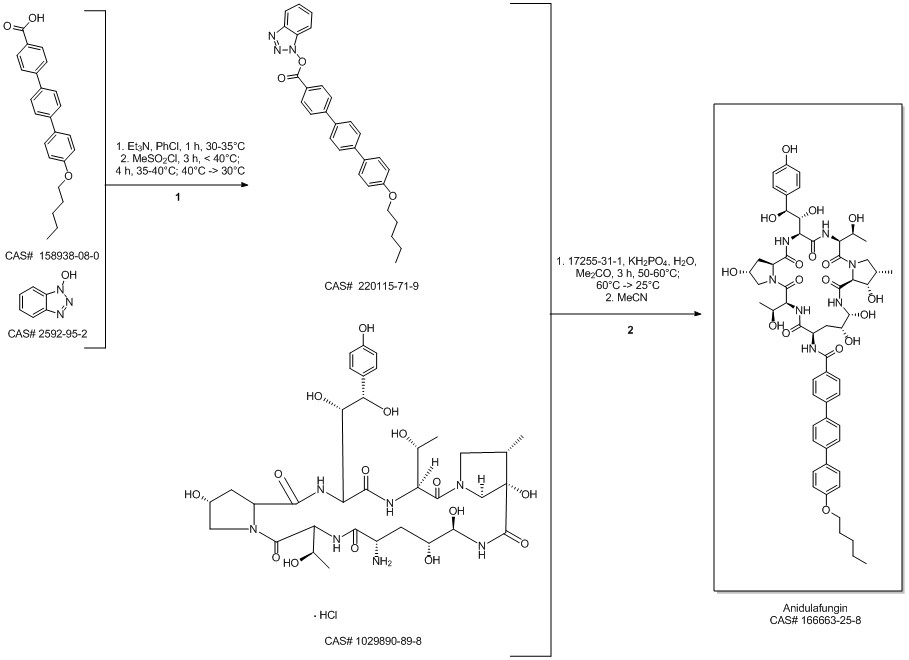
Norris, Timothy; VanAlsten, John; Hubbs, Stephen; Ewing, Marcus; Cai, Weiling; Jorgensen, Matthew L.; Bordner, Jon; Jensen, Grace O. Commercialization and Late-Stage Development of a Semisynthetic Antifungal API: Anidulafungin/D-Fructose (Eraxis). Organic Process Research & Development. Volume 12. Issue 3. Pages 447-455. 2008.

619-44-3
302 suppliers
$6.00/10g

166663-25-8
276 suppliers
$28.00/1mg
Yield:-
Steps:
Multi-step reaction with 4 steps
1: 1.) sec-butyllithium, 2.) triisopropyl borate, 3.) K2CO3, tetrakis(triphenylphosphine)palladium(0)
2: 2 N aq. NaOH / dioxane / 17 h / Heating
3: dicyclohexylcarbodiimide / CH2Cl2 / Ambient temperature
4: dimethylformamide / Ambient temperature
References:
Debono, Manuel;Turner, William W.;LaGrandeur, Lisa;Burkhadt, Fred J.;Nissen , Jeffrey S.;et al. [Journal of Medicinal Chemistry,1995,vol. 38,# 17,p. 3271 - 3281]
63619-51-2
29 suppliers
$9.00/250mg

166663-25-8
276 suppliers
$28.00/1mg
![[1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-](/CAS/GIF/158938-08-0.gif)
158938-08-0
96 suppliers
$15.00/250mg

166663-25-8
276 suppliers
$28.00/1mg
![4''-(Pentyloxy)-[1,1':4',1''-terphenyl]-4-carboxylic acid methyl ester](/CAS/20150408/GIF/158937-30-5.gif)
158937-30-5
7 suppliers
inquiry

166663-25-8
276 suppliers
$28.00/1mg