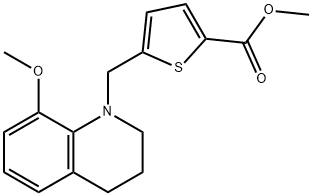
methyl 5-((8-methoxy-3,4-dihydroquinolin-1(2H)-yl)methyl)thiophene-2-carboxylate synthesis
- Product Name:methyl 5-((8-methoxy-3,4-dihydroquinolin-1(2H)-yl)methyl)thiophene-2-carboxylate
- CAS Number:1423018-14-7
- Molecular formula:C17H19NO3S
- Molecular Weight:317.4

53899-17-5
41 suppliers
$430.00/1g
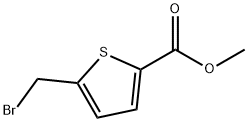
108499-32-7
37 suppliers
$45.00/5mg

1423018-14-7
6 suppliers
inquiry
Yield:1423018-14-7 56%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in ethanol at 20;
Steps:
5 Preparation 5 Methyl-5-[(8-methoxy-3,4-dihydro-2H-quinolin-l-yl)methyl]thiophene-2-carboxylate
Add methyl-5-(bromoethyl)thiophene-2-carboxylate (432.5 g, 1.84 mol) in EtOH (500 ml) to a solution of 8-methoxy-l,2,3,4-tetrahydroquinoline (300 g 1.84 mol) in EtOH (1 L) and stir. Add DIPEA (641 ml, 3.67 mol) dropwise and stir at room temperature overnight. After completion of the reaction, remove the EtOH in vacuo, and add water (5 L). Extract the aqueous with EtOAc (3 x 3 L); combine the organic layers; and dry over sodium sulfate. Filter the solution and concentrate under reduced pressure to give a residue. Purify the residue by silica gel flash chromatography eluting with ethyl acetate: hexane (6:94) to give the title compound (325 g, 56%). ESI (m/z) 318(M+H).
References:
WO2013/25424,2013,A1 Location in patent:Page/Page column 8
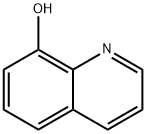
148-24-3
879 suppliers
$5.00/25g

1423018-14-7
6 suppliers
inquiry
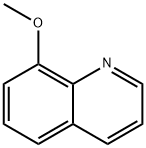
938-33-0
101 suppliers
$22.80/250mg

1423018-14-7
6 suppliers
inquiry
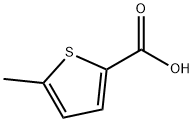
1918-79-2
243 suppliers
$8.00/5g

1423018-14-7
6 suppliers
inquiry

19432-69-0
68 suppliers
$24.00/250mg

1423018-14-7
6 suppliers
inquiry