
7-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carboxylic acid synthesis
- Product Name:7-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carboxylic acid
- CAS Number:1426135-67-2
- Molecular formula:C9H5F3N2O2
- Molecular Weight:230.14
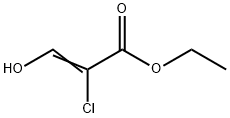
10229-12-6
2 suppliers
inquiry
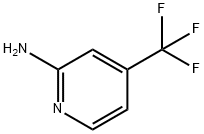
106447-97-6
344 suppliers
$11.00/1g
![7-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carboxylic acid](/CAS/20180702/GIF/1426135-67-2.gif)
1426135-67-2
21 suppliers
inquiry
Yield:1426135-67-2 93%
Reaction Conditions:
Stage #1: ethyl 2-chloro-3-hydroxyacrylate;2-amino-4-(trifluoromethyl)pyridine in ethanol at 80;Sealed tube;Inert atmosphere;
Stage #2: with water;lithium hydroxide in ethanol at 60; for 3 h;
Steps:
Intermediate 101: 7-(trifluoromethyl)imidazo[ 1 ,2-a]pyridine-3-carboxylic acid
A solution of 4-(trifluoromethyl)pyridin-2-amine (100 mg, 0.62 mmol) and ethyl 2- chloro-3-hydroxyacrylate (139 mg, 0.93 mmol) in EtOH (3 mL) in a sealed vial was stirred under N2 at 80 °C overnight. The reaction was cooled to rt. To the reaction wereadded water (0.5 mL) and LiOH (57.0 mg, 2.34 mmol). It was stirred at 60 °C for 3 h. The solvent was removed. The crude product was purified by reverse phase chromatography to provide Intermediate 101 (132 mg, 93% yield) as a white solid. ‘H NMR (400MHz, DMSO-d6) ? 9.43 (d, J=7.3 Hz, 1H), 8.42 (s, 1H), 8.32 - 8.22 (m, 1H), 7.49 (dd, J7.4, 1.9 Hz, 1H). LC-MS(ESI) m/z: 230.9 [M+H].
References:
WO2016/10950,2016,A1 Location in patent:Page/Page column 227
![7-Trifluoromethyl-imidazo[1,2-a]pyridine-3-carboxylic acid ethyl ester](/CAS/20180703/GIF/1397206-76-6.gif)
1397206-76-6
19 suppliers
inquiry
![7-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carboxylic acid](/CAS/20180702/GIF/1426135-67-2.gif)
1426135-67-2
21 suppliers
inquiry