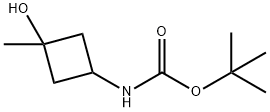
tert-butyl N-(3-hydroxy-3-methylcyclobutyl)carbamate synthesis
- Product Name:tert-butyl N-(3-hydroxy-3-methylcyclobutyl)carbamate
- CAS Number:1427329-27-8
- Molecular formula:C10H19NO3
- Molecular Weight:201.26
![(1-Oxa-spiro[2.3]hex-5-yl)-carbamic acid tert-butyl ester](/CAS/20210305/GIF/1154759-69-9.gif)
1154759-69-9
3 suppliers
inquiry

1427329-27-8
9 suppliers
inquiry
Yield:1427329-27-8 94%
Reaction Conditions:
with lithium triethylborohydride in tetrahydrofuran at 0; for 3 h;Inert atmosphere;
Steps:
D D. tert-Butyl (3-hydroxy-3-methylcyclobutyl)carbamate
Lithium triethylborohydride (34.8 mL, 34.8 mmol) in THE (1 .0 M) was added to tert-butyl 1-oxaspiro[2.3]hexan-5-ylcarbamate (Intermediate 21C) (5.34 g, 26.8 mmol) in THE (89 mL) at0 °C under nitrogen. After 3 h, reaction mixture was quenched with water, solid potassium carbonate was added, then extracted with diethyl ether, dried over magnesium sulfate, filtered, and concentrated. The residue was purified by silica gel chromatography, eluting with EtOAc:hexanes (2:3 to 1:1) to give the title compound (5.36 g, 94% yield). 1H NMR (400 MHz, CD3SOCD3) 1.17 (5, 1.5 H), 1.19 (5, 1.5 H), 1.35 (5, 9 H), 1.78-1.92 (m, 2 H), 2.08-2.18 (m, 2H), 3.46 (h, J = 8Hz, 0.5 H), 3.99 (h, J = 8Hz, 0.5 H), 4.70 (brs, 0.5 H), 4.83 (brs, 0.5 H), 7.00 (d, J = 6 Hz, I H); LC-MS (ES-MS) M+H = 202.
References:
WO2018/69863,2018,A1 Location in patent:Page/Page column 129
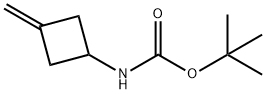
130369-04-9
61 suppliers
$49.47/250mgs:

1427329-27-8
9 suppliers
inquiry

15760-36-8
118 suppliers
$18.00/250mg

1427329-27-8
9 suppliers
inquiry
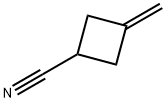
15760-35-7
136 suppliers
$10.00/1g

1427329-27-8
9 suppliers
inquiry