
3-acetyl-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one synthesis
- Product Name:3-acetyl-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one
- CAS Number:1438383-92-6
- Molecular formula:C19H16O3
- Molecular Weight:292.33
![5H-Benzo[d]naphtho[2,3-b]pyran-8(9H)-one, 10,11-dihydro-3-[2-(trimethylsilyl)ethynyl]-](/CAS/20210305/GIF/1438383-91-5.gif)
1438383-91-5
0 suppliers
inquiry
![3-acetyl-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one](/CAS/20200119/GIF/1438383-92-6.gif)
1438383-92-6
21 suppliers
inquiry
Yield:1438383-92-6 86%
Reaction Conditions:
with formic acid at 65; for 3 h;
Steps:
PY-3 3-acetyl-l 0,1 l-dihydro-5H-dibenzo [c,g] chromen-8(9H)-one
A 20 mL vial with stir bar was charged with 3-((trimethylsilyl)ethynyl)-10, l 1 -dihydro-5H- dibenzo[c,g]chromen-8(9H)-one (850 mg, 2.44 mmol) and formic acid (9.8 mL). The solution was heated to 65 °C. After 3 h, the reaction was judged complete. The mixture was concentrated under reduced pressure; the resulting residue was taken up in CH2CI2 and loaded onto a prepacked 25g silica gel cartridge. The product was purified by chromatography on a prepacked 80g silica gel column eluting with a solvent gradient from 5% to 85% EtOAc/hexanes. The product containing fractions were combined and concentrated to provide 3-acetyl-l 0, 1 l -dihydro-5H-dibenzo[c,g]chromen-8(9H)- one (616 mg, 86%): 400 MHz lH NMR (CDC13) δ 8.00 - 7.94 (m, 1H), 7.81 (d, J = 8.2 Hz, 1H), 7.77 (s, 1H), 7.64 (s, 2H), 5.16 (s, 2H), 2.98 (t, J = 6.1 Hz, 2H), 2.69 - 2.64 (m, 2H), 2.63 (s, 3H), 2.21 - 2.09 (m, 2H).
References:
WO2013/75029,2013,A1 Location in patent:Page/Page column 209; 210
![7-[(2-Bromo-5-chlorophenyl)methoxy]-3,4-dihydro-1(2H)-naphthalenone](/CAS/20150408/GIF/1378388-19-2.gif)
1378388-19-2
42 suppliers
$8.00/100mg
![3-acetyl-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one](/CAS/20200119/GIF/1438383-92-6.gif)
1438383-92-6
21 suppliers
inquiry
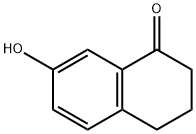
22009-38-7
207 suppliers
$5.00/250mg
![3-acetyl-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one](/CAS/20200119/GIF/1438383-92-6.gif)
1438383-92-6
21 suppliers
inquiry
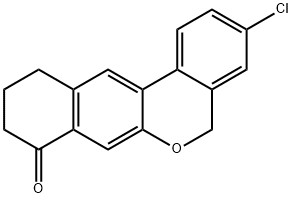
1378388-20-5
42 suppliers
$33.00/100mg
![3-acetyl-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one](/CAS/20200119/GIF/1438383-92-6.gif)
1438383-92-6
21 suppliers
inquiry
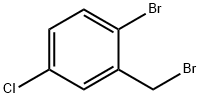
66192-24-3
118 suppliers
$9.00/1g
![3-acetyl-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one](/CAS/20200119/GIF/1438383-92-6.gif)
1438383-92-6
21 suppliers
inquiry