
ETHYL 4-METHYL-2-[3-(TRIFLUOROMETHYL)PHENYL]THIAZOLE-5-CARBOXYLAT synthesis
- Product Name:ETHYL 4-METHYL-2-[3-(TRIFLUOROMETHYL)PHENYL]THIAZOLE-5-CARBOXYLAT
- CAS Number:144061-14-3
- Molecular formula:C14H12F3NO2S
- Molecular Weight:315.31
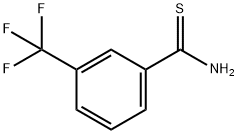
53515-17-6
63 suppliers
$24.89/250mg
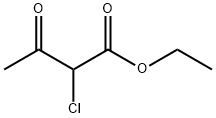
609-15-4
453 suppliers
$6.00/25g
![ETHYL 4-METHYL-2-[3-(TRIFLUOROMETHYL)PHENYL]THIAZOLE-5-CARBOXYLAT](/StructureFile/ChemBookStructure22/GIF/CB9653311.gif)
144061-14-3
18 suppliers
$50.00/100mg
Yield:144061-14-3 100%
Reaction Conditions:
in ethanol at 80;
Steps:
70a ethyl 4-methyl-2-[3-(trifluoromethyl)phenyl]-1,3-thiazole-5-carboxylate
ethyl 4-methyl-2-[3-(trifluoromethyl)phenyl]-1,3-thiazole-5-carboxylate To a solution (6 mL) of ethyl 2-chloroacetoacetate (220 mg, 1.3 mmol) in ethanol was added 3-trifluoromethylphenylthioamide (250 mg, 1.2 mmol), and the mixture was stirred at 80°C overnight. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The obtained organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure. The resulting crude title compound was purified by column chromatography (hexane - hexane/ethyl acetate=80/20) to give the title compound (380 mg, 100%) as a colorless solid. 1H NMR (300 MHz, CHLOROFORM-d) δppm 1.40 (3 H, t, J=7.2 Hz), 2.80 (3 H, s), 4.37 (2 H, q, J=7.2 Hz), 7.59 (1 H, t, J=7.8 Hz), 7.72 (1 H, d, J=7.7 Hz), 8.13 (1 H, d, J=7.9 Hz), 8.25 (1 H, s) LCMS (ESI+) M+H+: 316.
References:
EP2264017,2010,A1 Location in patent:Page/Page column 170

53515-17-6
63 suppliers
$24.89/250mg
638-07-3
500 suppliers
$5.00/5G
![ETHYL 4-METHYL-2-[3-(TRIFLUOROMETHYL)PHENYL]THIAZOLE-5-CARBOXYLAT](/StructureFile/ChemBookStructure22/GIF/CB9653311.gif)
144061-14-3
18 suppliers
$50.00/100mg

638-07-3
500 suppliers
$5.00/5G

53515-17-6
63 suppliers
$24.89/250mg
![ETHYL 4-METHYL-2-[3-(TRIFLUOROMETHYL)PHENYL]THIAZOLE-5-CARBOXYLAT](/StructureFile/ChemBookStructure22/GIF/CB9653311.gif)
144061-14-3
18 suppliers
$50.00/100mg
368-77-4
275 suppliers
$10.00/10g
![ETHYL 4-METHYL-2-[3-(TRIFLUOROMETHYL)PHENYL]THIAZOLE-5-CARBOXYLAT](/StructureFile/ChemBookStructure22/GIF/CB9653311.gif)
144061-14-3
18 suppliers
$50.00/100mg

1801-10-1
112 suppliers
$6.00/1g
![ETHYL 4-METHYL-2-[3-(TRIFLUOROMETHYL)PHENYL]THIAZOLE-5-CARBOXYLAT](/StructureFile/ChemBookStructure22/GIF/CB9653311.gif)
144061-14-3
18 suppliers
$50.00/100mg