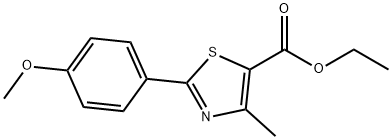
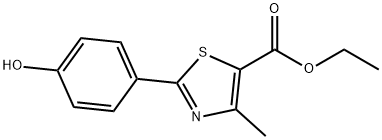
2-(4-Methoxy-phenyl)-4-methyl-thiazole-5-carboxylicacidethylester synthesis
- Product Name:2-(4-Methoxy-phenyl)-4-methyl-thiazole-5-carboxylicacidethylester
- CAS Number:54032-88-1
- Molecular formula:C14H15NO3S
- Molecular Weight:277.34
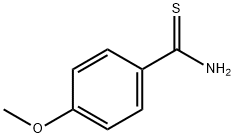
2362-64-3
108 suppliers
$10.00/1g
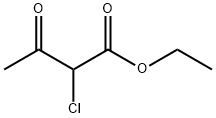
609-15-4
446 suppliers
$6.00/25g

54032-88-1
26 suppliers
$108.75/1g
Yield:54032-88-1 92%
Reaction Conditions:
in ethanol at 70; for 6 h;
Steps:
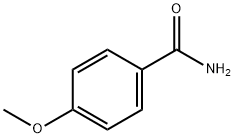
1 Ethyl 2-(4-methoxyphenyl)-4-methylthiazole-5-carboxylate (T7):
Add 4-methoxybenzamide (152.2 mg, 1 mmol) to a 25 mL round bottom flask.Lawesen reagent (485.4mg, 1.2mmol) and THF (20mL) were reacted at room temperature for 4h, then the Lawson reagent and solvent were removed, and the intermediate thiobenzamide was obtained by column chromatography.Then, it was refluxed with ethyl 2-chloroacetoacetate (329.2 mg, 2 mmol) in a solvent ethanol (20 mL) at 70 ° C for 6 hours. After completion of the reaction, the solvent was removed and purified by column chromatography to give Compound T7. Yield 92%, white solid, unfolded system PE/EA 10:1 (Rf = 0.5, PE / EA = 3:1)
References:
CN109810071,2019,A Location in patent:Paragraph 0061; 0072; 0073

2362-64-3
108 suppliers
$10.00/1g
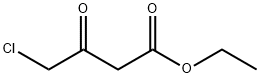
638-07-3
478 suppliers
$5.00/5G

54032-88-1
26 suppliers
$108.75/1g

2362-64-3
108 suppliers
$10.00/1g

638-07-3
478 suppliers
$5.00/5G

54001-16-0
27 suppliers
$24.19/250mg

54032-88-1
26 suppliers
$108.75/1g
161797-99-5
362 suppliers
$10.00/1g

74-88-4
342 suppliers
$15.00/10g

54032-88-1
26 suppliers
$108.75/1g
3424-93-9
199 suppliers
$14.00/1g

54032-88-1
26 suppliers
$108.75/1g