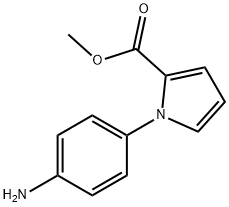
1-(4-Amino-phenyl)-1H-pyrrole-2-carboxylic acid methyl ester synthesis
- Product Name:1-(4-Amino-phenyl)-1H-pyrrole-2-carboxylic acid methyl ester
- CAS Number:1445153-72-9
- Molecular formula:C12H12N2O2
- Molecular Weight:216.24
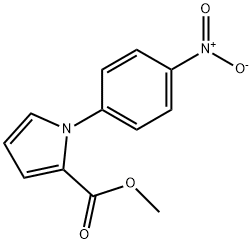
124475-67-8
12 suppliers
inquiry

1445153-72-9
3 suppliers
inquiry
Yield:1445153-72-9 480 mg
Reaction Conditions:
with sodium dithionite;water in ethanol; for 0.166667 h;Reflux;
Steps:
Methyl l-(4-aminophenyl)pyrrole-2-carboxylate (ii):
Sodium hydrosulphite (2.08 g, 10.15 mmoll) in water (7.5 mL) was added to a refluxing solution of (i) (1 g, 4.06 mmol) in EtOH (15 mL). After 5 minutes another solution of sodium hydrosulphite (2.08 g, 10.15 mmoll) in water (7.5 mL) was added. The mixture refluxed for 5 min. After that time the mixture was poured onto water. The reaction mixture was extracted with EtOAc and DCM/iPrOH. The organic fractions were dried (MgS04), filtered and concentrated in vacuo to give a residue that was purified by flash chromatography on silica gel, eluting with a MeOH gradient in DCM to afford the desired product (ii) (480 mg). *H NMR (400 MHz, DMSO) δ 7.06 (dd, J = 2.5, 1.9 Hz, 1H), 6.96 - 6.90 (m, 3H), 6.59 - 6.53 (m, 2H), 6.22 (dd, / = 3.9, 2.6 Hz, 1H), 5.25 (s, 2H), 3.60 (s, 3H).
References:
WO2013/91011,2013,A1 Location in patent:Page/Page column 61
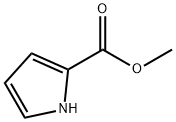
1193-62-0
222 suppliers
$5.00/1g
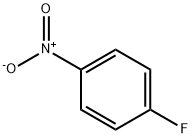
350-46-9
516 suppliers
$10.60/1gm:

1445153-72-9
3 suppliers
inquiry